

Figure 23.-The California red-backed vole depends on decayed fallen trees as habitat (photo, courtesy of D. Ure).

Figure 24.-Fruiting bodies of Rhizopogon vinicolor A. H. Smith: left and right, surface views; center, cross sections. This fungus typically forms mycorrhizae with Douglas-fir roots in rotten wood.

Figure 25.-Mycorrhizae of Douglas-fir formed with the fungus Rizopogon vinicolor.

Redbellied checkered beetle (Above)
Animal-animal.-As a class I fallen tree is penetrated by the wood-boring beetles and they begin to thrive within it, Nature’s system of checks and balances is also activated. At first this system is composed primarily of predaceous beetles in the families Cleridae (checkered beetles) and Trogositidae (no common name). The redbellied checkered beetle, for example, is an important predator of the bark beetle in Douglas-fir trees (Cowan and Nagel1965). Adult redbellied checkered beetles prey on adult bark beetles, and their larvae prey on the larvae of bark beetles. There is one generation of redbellied checkered beetles annually in Oregon (Furniss and Carolin 1977).
As the diversity of fauna within a fallen tree increases, so do the number and variety of predators. Among the smallest predators are the predaceous mites. Predaceous mites are common near the surface of the soil and in mosses, humus, rotten wood, and animal waste products. They prey on small arthropods, such as collembolans, on arthropod eggs, on small roundworms, and occasionally on each other. Predaceous mites are commonly long legged and fast, and they have strong mouthparts for capturing and chewing their prey (Krantz 1978, Luxton 1972).

Pseudoscorpions (Above)
The next level of predation may be pseudoscorpions. These little creatures are related to spiders. They look like miniature scorpions with pinchers but without tails and stingers.
Page 32
Pseudoscorpions move in a slow walk, but they can climb and can walk upside down on the ceilings of small caverns within rotten wood; they move backward much faster than forward. They construct silken nests in which they rest. A pseudoscorpion not occupied in spinning silk often sits in its nest with its pinchers (technically called pedipalps) and the anterior of its body protruding through the opening. From this position, it can grab a passing collembolan or mite to eat. Some species of pseudoscorpions seem to prefer small flies, small beetles and their larvae, ants, and even small earthworms (Comstock 1948, Weygoldt 1969). The pseudoscorpion keeps its nest clean, discarding prey remains as far away from its nest as possible without actually leaving the nest. An individual may leave its nest in search of food or a mate; when it cannot find its way back, it makes a new nest or occupies an empty one (Weygoldt 1969).
A female pseudoscorpion carries its eggs and developing embryos in a brood sac attached at its genital opening. The eggs are small, with little yolk, so the embryos are nourished by a nutritive fluid produced by the female. Most species reproduce in spring or summer and may have several broods per season (Weygoldt 1969).

Folding-door spider (Above)
The next order of predaceous magnitude is probably spiders, of which the Pacific folding-door spider is the largest spider inhabiting fallen trees. In general, spiders undergo little metamorphosis or change during development; when hatched, they look like miniature adults. Legs lost during development are usually regenerated. Although spider eggs hatch soon after they are laid, spiderlings that hatch in the autumn remain in the brood sac until the next spring. All spiders are predaceous; they eat mainly insects. A spider normally kills its prey by injecting poison into the captured prey with its "fangs" (Borror and DeLong 1964, Comstock 1948).
The folding-door spider’s abode is a tube constructed in an existing crack within the outer layer of a class III or IV fallen tree with many cracks and crevices. The horizontal tube is completely lined with silk. To close its tube, a spider grasps the rim on opposite sides and pulls it in toward the middle. Except when capturing prey, a female seldom leaves her tube, but a male wanders in search of a mate (Levi and others 1968).
From our observations, Pacific folding-door spiders prey on whatever they can catch and subdue. Although we have occasionally found evidence of food refuse around the entrance of a spider’s tube, it was usually scattered as we dissected a fallen tree and was not identifiable.
Another group of predators is the centipedes, of which Scolopocryptops sexspinosa (Say) (no common name) is the largest found in fallen Douglas-firs. Centipedes have one pair of legs per segment. Centipedes overwinter as adults in a protected place, such as within rotten wood. They lay their eggs in the spring and early summer. Some species have sticky eggs that a female hides with debris, but a female S. sexspinosa usually coils around her eggs to protect them and may periodically lick them to keep them clean (Borror, and DeLong 1964, Levi and others 1968).
Centipedes are predaceous. They feed on spiders, insects, and other small animals. All centipedes have poison jaws with which they paralyze their prey. Some species, such as S. sexspinosa, have such strong poison jaws that they are effective even against such predators as birds and large insects (Borror and DeLong 1964, Levi and others 1968, Maser and Hooven 1974). Centipedes figure prominently as predators in trees in decay classes III, IV, and V.
Page 33
As the bark becomes loose on a late class II fallen tree, lungless salamanders (family Plethodontidae) join the internal community. Three species of salamanders are associated, as predators, with rotten wood in western Oregon: Oregon slender salamander, Oregon salamander, and clouded salamander.
Oregon slender salamanders are endemic to the northern half of the Cascade Range in western Oregon (Stebbins 1966). They are most often associated with trees in decay classes III to V, either under intact bark or in termite channels deep within (Stebbins 1954). Females lay about 8 to 11 eggs in June. Large, moist, cool fallen trees are important to the Oregon slender salamander in the heat of summer because they, and other species of slender salamanders, seem to be particularly prone to fatality from heat stress (Maiorana 1977). They may actually need a suitable fallen tree for only a few weeks in summer, but without it during that time they could die.
Although we know of no specific data on the food habits of the Oregon slender salamander, a similar species that occurs in extreme southwestern Oregon and in northwestern California, the California slender salamander, will serve as an example. Both species are about the same size and inhabit forested areas. The major items in the diet of California slender salamanders are collembolans and mites, followed by such groups as flies, spiders, and small snails (Bury and Martin 1973). Evidence suggests that the California slender salamander even selects collembolans (specifically family Sminthuridae) and mites (specifically family Oribatidae) over other types of prey (Maiorana 1978). These small, slender salamanders are well suited to a role of predator within the narrow confines of wood-boring beetle and termite galleries in classes III through V fallen trees.
Another salamander, the Oregon salamander, although thought to primarily inhabit rodent burrows in forested areas (Stebbins 1954), is frequently found under pieces of bark that sloughed off large, fallen Douglas-firs. It is also found within classes III through V trees, particularly during cold or dry weather (Stebbins 1966). Individuals are usually solitary except when they are breeding or are associated with young. Egg clutches, averaging 11 or 12 eggs, are often deposited under the bark or within fallen, rotting Douglas-firs. One female with a clutch of 12 eggs was found on June 30, 1982, in a very wet class IV Douglas-fir in an old-growth Douglas-fir stand on Mary’s Peak, Benton County, Oregon.
The two most important foods of the Oregon salamander are collembolans and spiders, followed by isopods (sowbugs), millipedes, and adult beetles (Bury and Martin 1973).
The third salamander is the clouded salamander (fig. 19). It frequents rotten wood, particularly Douglas-fir in late classes II through IV (Stebbins 1966). These salamanders are often found under the loose bark of large fallen trees in spaces excavated by wood-eating insects (Fitch 1936). In fact, young clouded salamanders show a striking affinity for bark (McKenzie and Storm 1970). According to Stebbins (1954) they are especially abundant in well-illuminated openings in a forest. In addition, the clouded salamander is the most arboreal member of the genus and has been found 20 feet (6.1 m) up in standing trees (Stebbins 1954).
Eggs laid in late spring or early summer under bark and in cavities in rotten wood are guarded by the female. The eggs may be attached separately by their stalks but close together, or they may have their stalks twisted around one another and be attached to a common point on the ceiling or wall of the nesting chamber (Stebbins 1954).
Page 34
 I
I
Bury and Martin (1973) and Storm and Aller (1947) listed ants as the most important food of clouded salamanders. We also found ants to be an important food item, but only in summer.
² The major food of the adult clouded salamanders in winter, spring, and fall were isopods and beetles, particularly snout beetles. Isopods, ants, beetles, and common earwigs were important in summer, when foods were eaten in greater diversity. Important foods for small juvenile salamanders (as large as three-fourths inch (19 mm) in snout-vent length) were mites, collembolans, flies, and very small beetles. Larger juveniles (more than three-fourths inch (20 mm) in snout-vent length) consumed, in order of importance, flies, isopods, beetles, mites, and centipedes in winter; beetles, ants, and isopods in spring; ants and beetles in summer; and isopods, beetles, and ants in fall (see footnote 2). Storm and Aller (1947) also found termites, probably the Pacific dampwood termite, in the stomachs of some individuals, which indicated that the salamanders had been feeding within a class III to class IV fallen tree.

The final level of predation within large, rotten, fallen Douglas-firs in classes III through V is probably that of small mammals, such as shrews and shrew-moles.
Shrews are small, with short legs, tiny eyes, and long, pointed noses. Although they cannot see well, their senses of touch, smell, and hearing are acute. The common shrew in western Oregon Douglas-fir forests is the Trowbridge shrew. This small, "nervous" mammal is abundant around fallen trees, particularly classes III and IV, that are well settled on the forest floor and have been in place long enough to act as the shrew’s grocery. The Trowbridge shrew has the most catholic diet of all western Oregon shrews. It eats at least 47 types of food, the most important of which are centipedes, spiders, internal organs of invertebrates (probably mostly beetles), slugs, and snails. In addition, it shows a definite affinity for fallen trees, as do some of its prey (Maser and others 1981, Terry 1981, Whitaker and Maser 1976).
The American shrew-mole is a tiny mole. As are other moles’ ears, the shrew-mole’s ears are merely holes near the shoulders and are not visible because of the dense fur. It has minute eyes nearly concealed by fur, and broad front feet with stout claws adapted for digging (Maser and others 1981).
These small moles spend much time burrowing in the surface soil-litter layer and along and under classes II and III fallen trees. When trees reach classes IV and V, shrew-moles also burrow within them. Their close tie with fallen trees in old-growth forests is probably reflected in their diet (Maser and others 1981, Terry 1981); for example, in a study by Whitaker and others (1979), the three foods eaten with the highest frequency were earthworms (81.8 percent), centipedes (54.5 percent), and flies (36.4 percent).
The shrew-mole is ideally equipped to forage in and around fallen trees because its nose is extremely sensitive to touch; it is much like a blindman’s cane. In almost constant motion, it quickly identifies any object it contacts. Further, this mole’s small size, adaptions for digging, and herculean strength make it an efficient, burrowing predator within and beneath rotten wood (Maser and others 1981).
- - - - - - - - - - - - - - - -
² J. O. Whitaker, Jr., and C. Maser. Unpublished data on file at Indiana State University, Department of Life Sciences, Terre Haute, Indiana 47809.
Page 35
Nutrient-plant-animal-nutrient.- A tree begins life with nutrients from the soil and ends life with nutrients that diffuse into the forest floor and become parts of other trees. It is appropriate, therefore, to select an element (nitrogen) that is necessary for the growth and reproduction of all organisms within a forest and to use it to represent full cycle the minisystems within a fallen tree.
As a tree grows, nitrogen is incorporated into the wood-cell structure. After it dies, processes of decomposition begin to break down the structure of the wood and ultimately to recycle the nitrogen.
During decomposition, micro-organisms (such as fungi and bacteria) incorporate nitrogen from the wood into their own cellular structures as they digest the carbon from the wood substrates. As the microbes decompose wood, the carbon-to-nitrogen ratio gradually shifts until nitrogen becomes relatively more available for plant uptake. As microbial colonization and decay increase, animals varying in size from small mites and collembolans to large beetle larvae feed on microbial tissues enriched in nitrogen from digestion of wood; and vertebrates, from California red-backed voles to black-tailed deer, may obtain some of their protein nitrogen from decaying trees by feeding on fungal fruiting bodies, such as truffles and mushrooms (Fogel and Trappe 1978, Maser and others 1978a, Trappe and Maser 1978).
As decomposition proceeds in a fallen tree, other sources of nitrogen are added in the form of leaf litter and other litter components that fall on the surface of the tree. As these decay, their nitrogen becomes available to enrich the wood; and as rainwater-rich in nutrients from passing through the tree canopy and surface litter-accumulates on the fallen tree’s upper surface, some nitrogen is leached into the wood.
Microbes that colonize a fallen tree from the ground surface provide additional nitrogen, as do animals that take up residence in the tree or eliminate their metabolic waste products on its upper surface. As decay proceeds, plant roots penetrate the fallen tree’s surface and tap the rotting wood as a source of available nitrogen, other nutrients, and water.
A fallen tree disappears gradually through the decades, and its nitrogen capital is returned to the forest. Weathering processes, such as freezing and thawing, and animal activities contribute to the disintegration and disappearance of the tree. Some residue may remain for centuries in the forest as it slowly becomes incorporated into the organic portion of the soil (fig. 20).
Biotic Succession
Within and Around
Fallen Trees
As a fallen tree decomposes, it creates a gradually changing myriad of internal and external habitats (Maser and others 1979). Plant and animal communities within a fallen tree are very different from those outside, but both progress through a series of orderly changes. As a fallen tree decomposes, its internal structure becomes simpler, whereas the structure of the plant community surrounding the fallen tree becomes more complex (figs. 26 and 27).
Page 36

Figure 26.-Plant and animal communities are very different inside this class Ill Douglas-fir than they are outside. (Above)
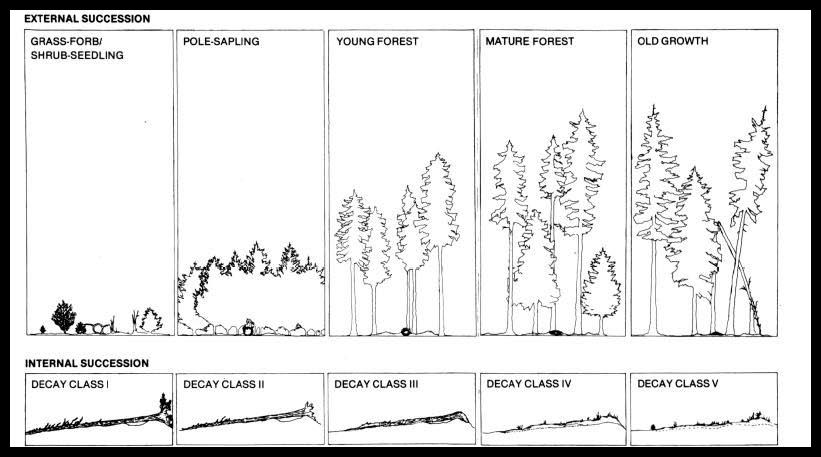
Figure27.-Fallen trees progress through two simultaneous successional processes-internal and external (modified from Maser and others 1979). (Above)
Internal succession in a fallen tree is related to the following factors: (1) the tree species and its inherent decay-resistant chemical properties; (2) its size-the larger it is, the longer it lasts; (3) what killed the tree; (4) whether it originated as a tree or a snag; (5) the microclimate around it; (6) its placement on the ground; and (7) the biotic community peculiar to it.
Internal succession is also influenced by temperature, moisture, and stage of decay. A class I fallen tree, for example, has many readily available nutrients that support opportunistic colonizers. As decay proceeds, its moisture-holding capacity increases, but nutrients become less available because either they have been used or they remain locked in the more decay-resistant compounds of the wood. Ultimately, the rapidly growing opportunists are succeeded by organisms with more sophisticated enzyme systems, and decay continues.
Page 37
External succession is related to the changes that take place in the plant community surrounding a fallen tree. A fallen tree is a connector between the successional stages of a community; it provides continuity of habitat from the previous forest through subsequent successional stages. A large fallen tree therefore provides a physical link-a nutrient savings account-through time and across successional stages. Because of its persistence, a fallen tree provides a long-term, stable structure on which some animal (both invertebrate and vertebrate) populations appear to depend for survival.
External succession is influenced by the same factors as internal succession, with the additional influence of light. Consider, for instance, a class IV or V tree that supports a lush community of mosses, liverworts, hemlocks, and other flowering plants. As the canopy closes over the opening created by the original falling of the tree, light becomes limiting to the growth of green plants. If, at this point, a nearby tree falls, the environment can change immediately and strikingly. Greater solar radiation increases the amount of light but may also raise the daytime temperature of the fallen tree. In turn, nighttime temperature may be lower because of the increased heat that reradiates to the atmosphere. In addition, more rain and snow reach the ground.
Stream Characteristics
and Fallen Trees
Fallen trees and other large pieces of wood significantly shape the energy flow, nutrient
dynamics, and structure of biota in streams of the Douglas-fir region (fig. 28). Streams in old-growth forests contain large quantities of organic debris: 220 to 770 tons per acre (200 to 700 t/ha). Large, organic debris shapes a stream channel by damming it, which creates ponds that trap sediments, or by obstructing it, which redirects waterflow that creates meanders and pools (Anderson and others 1978, Franklin and others 1981, Naiman and Sedell 1979, Sedell and others 1982b, Swanson and others 1976, Triska and Cromack 1980, Triska and others 1982).Large pieces of wood in streams provide a diversity of spawning and rearing habitats for salmonids. In the smallest (first order) streams, for example, over 50 percent of the habitat is related to presence of large wood (fig. 29) and about 25 percent is created and maintained by wood in larger (third order) streams (Anderson and Sedell 1979, Franklin and others 1981, Swanson and Lienkaemper 1978, Triska and others 1982).
Most large wood is randomly spaced in small streams (first and second order) because flow volume is insufficient to transport large trees downstream. Intermediate streams (third to fifth order) have less wood. Big chunks typically accumulate where the channel is obstructed by immobile dead trees or channel constrictions; such obstructions provide sites for collection of small to intermediate size debris that moves downstream at high flows. Most debris comes to rest on the flood plains or on the outsides of bends of larger streams or rivers (sixth to eighth order). But even in big rivers, historical records show that large pieces of wood contribute significantly to in-channel structures that trap sediment, pond water, and create side channels and sloughs (Franklin and others 1981, Naiman and Sedell 1979, Sedell and Luchessa 1982, Swanson and others 1978, Swanson and Lienkaemper 1978).
The food base or energy supply of a stream in an old-growth Douglas-fir forest is primarily litter from the adjacent forest combined with algae produced in reaches of streams exposed to light. Pristine streams retain much of the forest litter. Fallen trees in a stream form "stairsteps" that allow over 70 percent of the litter to be retained long enough to be biologically processed by stream organisms. Similar relationships exist for pristine streams in the Canadian boreal forest (Naiman 1982, Sedell and others 1975, Triskaand others 1982).
Page 38

Figure 28.-Note how the fallen tree (foreground) channels the water against the rootwad (left) that, in turn, buffers the stream bank from current. (Above)

Figure 29.-The stable, fallen tree forms an obstruction that dissipates some of the stream's energy and creates a pool used by salmonids. (Above)
The influence of the forest as a source of energy and as a channel structure diminishes as a stream gets larger. Edges of an unmanaged stream, however, are dominated by forest vegetation, and fallen trees create and maintain side channels and small backwater areas that are prime sites for deposition of organic materials and rearing of coho salmon (Sedell and others 1982a). Further, old-growth forests typically have a mix of herbaceous and shrubby plants and areas of various sizes exposed to sunlight; this combination provides a stream with a mix of coniferous and deciduous leaf litter as well as patches of algae. Such diversity of habitat and energy provides an interactive aquatic system with a stable, rich mix of both invertebrate and vertebrate species.
A Historical Sketch of
Woody Debris in
Northwest Waterways
Most early descriptions of Northwest streams and rivers are recorded in British and United States army journals. They tell, for example, of valleys so wet that early travel was along the edges of the hills and not along the valley bottoms (Dicken and Dicken 1979). The Tualatin Valley was described in British army journals as "mostly water connected by swamps" (Ogden 1961, p. 122). Much of this flooding was a result of beaver activity and accumulated sediment, fallen trees, and living vegetation in the channels. Because the valley bottoms had accumulated fine silt and organic material of alluvial origin, the land was fertile, and the task of draining it for farming began early in Oregon and Washington.
Oregon State Agricultural College soils scientist, I. A. Williams (1914, p. 13), wrote of the condition of Willamette Valley streams in 1910:
Many of the smaller streams. . . through these flat sections of the valley flow sluggishly and frequently overflow their banks during periods of heavy winter rainfall. . . . Most of these have sufficient grade to carry even more water than ordinarily comes to them; seldom less than 3, and usually more, feet of fall per mile. The annual overflow is caused from the obstructing of the channel by the growth of trees and the extension of their roots, the dams thrown across the channels by beavers and the consequent accumulation of sediment and other debris, etc. . . . It is a common condition, however, and usually all that is necessary is a clearing out and opening up of the clogged channel of the stream to afford entire relief . . . to the farmer. . . .
Page 39
Descriptions of streams in the Puget Sound lowland and the Willamette Valley were similar. Most consisted of a network of sloughs, islands, beaver ponds, and driftwood dams with no main channel. The Skagit River lowlands encompass about 198 square miles (512 km
²), of which over 50 square miles (128 km²) were marsh, sloughs, and wet grass meadows. U.S. Army Corps of Engineer maps for 1875-91 for the lower Nooksack and Snohomish Rivers in Washington show large areas of sloughs, swamps, and grass marshes (Reports of the Secretary of War 1875-99). All the coastal Oregon valleys contained marshy areas and numerous sloughs. The interaction of the streams and their flood plains in the lowlands of both States was great before they were cleared and channeled by pioneer farmers (U.S. Congress, House 1848).The channels of fast, turbulent rivers and low-gradient rivers, regardless of alluvial or bedrock control, were influenced by large amounts of wood. The lower Siuslaw River and lower North Fork Siuslaw River were so filled with fallen trees that trappers were unable to explore much of these river systems in 1826 (Ogden 1961). The Willamette River flowed in five separate channels between Corvallis and Eugene in 1870.
Reports of the Secretary of War (1875-99) state that the "obstacles were . . . great above Corvallis" and the riverbanks were heavily timbered for half a mile (0.8 km) on either side. Over 5,500 drifted, dead trees were pulled from a 50-mile (80-km) stretch of river in a 10-year period. The trees ranged from 5 to 9 feet (1.5 to 2.8 m) in diameter and from 90 to 120 feet (27.7 to 37 m) in length. The river was also confined to one channel by engineering activities. In both Oregon and Washington, other rivers were completely blocked by driftwood in the lower, main channels. The Skagit River, for example, had a driftwood jam that was three-fourths of a mile (1.2 km) long and one-fourth of a mile (0.4 km) wide. The Stillaguamish River was closed by six driftwood jams from the head of tidewater to river mile 17 (km 27.2). Drifted, dead trees were so numerous, large, and deeply imbedded in the bottom that a steam "snag boat" had to operate for 6 months to open a channel 100 feet (30 m) wide.
Driftwood jams in high-gradient river systems were often located where the channel gradient abruptly decreased. Morse (1883, p. 9) described the South Fork Nooksack River:
. . . we came to a place where the river, during freshets had ground sluiced all the earth away from the roots of the trees, and down some 6 feet to the gravel. This covered a region of country a mile in width by five in length. Overgrown yellow fir timber had once covered most of that section. If the river below there was only clear of jams that place would be a paradise of hand loggers. On the gravel lay many million feet of sound fir timber, which only needed to be junked up during the summer and the winter freshets would float the logs down to the sea. Immediately below this place, the jams first extend clear across the river, and for the next 20 miles there is a jam across the river nearly every mile.
From the above scenario, it is obvious that large, woody debris was an important factor in the early river systems of the Pacific Northwest (figs. 30 and 31). Human objectives, other than watershed management, however, dictated clearing the rivers; so present practices downplay the ecological role of large, woody debris in modern river systems.
Page 40

Figure 30.-Logs in a small stream awaiting enough water to be floated to the mill (photo from USDA Forest Service historical files. (Above)

Figure 31.-A splash dam at Austin Place, Hamilton Creek, Oregon, August 16, 1907. Such dams were used to regulate the flow of water to float logs down a stream to the mill. (Historical photo, courtesy of the Horner Museum, Oregon State University.) (Above)

Figure 32.-Note the large pieces of wood on the flood plain, especially in the lower right corner where the stable wood has formed a protected site that has allowed alder to grow.
Vegetation of Streamsides
and Gravel Bars
Large, fallen trees have both positive and negative effects on live vegetation that borders watercourses. Trees carried by floodwaters can severely batter live plants on a flood plain, but this is normally restricted to a narrow belt along the immediate channel. Stabilized, large pieces of wood, on the other hand, provide protected sites where alder and other species of plants can become established (fig. 32).
Once established, live vegetation begins to stabilize a stream channel. Such features as a gravel bar also become stabilized and enriched with fine sediments and organic materials as plant root systems develop and the stems resist the flow of water and reduce its velocity.
Fallen trees protect thickets of vegetation on exposed channel bars. Alders growing in bordering areas not protected by down trees sustain heavy, repeated pruning by floating woody debris and moving bedloads (Swanson and Lienkaemper 1982). The down trees that protect the outer edge of a thicket and those in the thicket itself create local environments of quiet water where fine sediments and organic debris are deposited during high flows. This process, coupled with the production of leaf and woody litter by the stand, results in soil development and growth of the stand. The large, down trees thus help a stand to reach the stage of structural development that allows it to better withstand floods.
Page 41
Fallen trees on gravel bars also provide sites where some stream-transported species of hardwoods and shrubs can reroot and grow.
Restabilization of streams after major floods, debris torrents, or massive landslides is accelerated by the presence of large, woody debris along and within a channel. Swanson and Lienkaemper (1978) found that after a fire an aquatic habitat was maintained by the large, woody debris (supplied to the stream by the prefire forest) while the postfire forest was developing. In many instances, however, salvage logging of streamsides destabilizes the structure of the channel and thereby the quality of the habitat.
Decomposition and
Nutrients in Streams
Small streams that drain heavily forested watersheds depend on organic materials from the adjacent forest as a source of carbon and nutrients for biological processes. Energy flow and nutrient cycling are measured by calculating the budgets of carbon and nitrogen in a stream. By examining the internal transfer of organic materials and nutrients, we can gain significant insights on the ability of a small stream to process incoming sources of energy. Sedell and others (1974) and Triska and others (1982) studied a small stream in the western Cascade Range of Oregon. They found that 85 percent of the organic material in the stream was large, fallen trees or branches from large trees. Of the tree leaves and needles that fell into the stream during the year, only 10 to 20 percent were transported downstream. The remainder were either stored in the channel or used as food by microbes. Microbial respiration accounted for about 55 percent of the carbon produced. There also was a large amount of fine detritus that was partly formed as a byproduct of the decomposition of large pieces of wood.
Large wood was the primary structural feature of small streams, and it dominated the carbon budget. The structural characteristics of large wood allowed leaves and needles to be retained in a stream long enough to be used as food by microbes and invertebrates. Without the wood, such material would be rapidly transported downstream, and the stream system would not be as efficient in processing organic material. The ability of a small stream to process organic material is also important because the stream provides preconditioned food to biotic communities in larger streams and rivers.
Retention of organic material is important to a stream ecosystem in that large quantities of stored organic material buffer the annual fluctuations in energy flow. During periods of drought, for example, enough moisture and waterlogged material remain available in a stream to provide habitat and food for aquatic organisms; during major floods when large amounts of organic material are transported downstream or washed up on the banks, enough remains to continue to provide habitat and food for aquatic organisms.
It is important to understand that carbon, nitrogen, and all the other materials that leave a watershed either pass through or accumulate in the stream environment, which encompasses less than 1 percent of the watershed area. Such a concentration of nutrients, the capacity of a stream to store organic material, and the efficiency to process it depend on the number and quality of fallen trees in the stream.
Page 42

Figure 33.-Some of these fallen trees will become water logged; others will be submerged only during high water. Both circumstances affect the rate of decoposition.
Decomposition and nutrient cycling.- Trees that fall into streams decompose at different rates and in different patterns than those that fall on the ground. Decomposition in water is slower than on land because waterlogging prevents deep diffusion of oxygen into the wood; the fungi and invertebrates that cause the most rapid decomposition of fallen trees on land are strongly aerobic (Triska and Cromack 1980). Waterlogged parts of fallen trees tend to decompose in thin surface layers, about one-fourth inch (0.5 cm) thick. As the decomposed surface is grazed or abraded, oxygen can penetrate farther into the underlying wood, which in turn becomes substrate for the decomposers. If only part of a fallen tree is in constant contact with water, that part decomposes slowly but the exposed part may decompose quite rapidly because neither a low level of oxygen nor extremely high or low moisture content limits decomposer activity. Trees that fall in very small streams may contact water only during the rainy season when the stream is flowing at its highest, and lack of moisture the rest of the year may slow their decomposition (fig. 33).
As decomposition of large pieces of wood advances, the concentration of essential nutrients, such as nitrogen and phosphorous, increases. Nitrogen increases primarily through biotic use of the carbon and through fixation of nitrogen. Nitrogen-fixing micro-organisms use both the wood and the bark of a fallen tree. Although bark decays more slowly than wood, the tannins in Douglas-fir bark are not effective in reducing nitrogen fixation (Baker and others 1983). Nitrogen fixation on fallen trees in streams accounts for 5 to 10 percent of the annual nitrogen supply to the stream (Triska and others 1982).
Page 43
Aquatic Invertebrates
Midge adult (Above)
The continuum of animal associations on woody debris in aquatic systems varies from being restricted to the wood to using it opportunistically. The sequence of colonists parallels the stage of wood decay. New wood entering a stream is used primarily as habitat, although some species of midges (Chironomidae) tunnel in the cambium and phloem. The wood is then colonized by a community of algae and microbes that provides food for a group of insects, functionally called grazers or collectors. Although this type of feeding does not significantly affect the structure of the wood, the colonization of the superficial layer of the wood by fungi softens it enough that it may be abraded and ingested by invertebrates that scrape their food off surfaces. Most important, however, the wood becomes suitable for obligate wood grazers and the more generalized wood shredders, such as caddisflies (Trichoptera) and stoneflies (Plecopteia), that ingest the wood infested by fungi. These activities result in a more sculptured surface texture that in turn provides habitat for many organisms. Decades of fungal colonization and growth soften the wood and allow oxygen to penetrate. Invertebrates that bore into the internal matrix of a fallen tree speed the decomposition process by consuming the wood and by transporting microbes into the tree. In the final phase of decay, detritivores-such as annelid worms (earthworms)-penetrate the material; continued decomposition then resembles that in soil and damp, terrestrial habitats (Anderson and others 1978, Dudley and Anderson 1982).
In contrast to sound wood that enters a small stream as described above, some wood is already conditioned by fungi and other terrestrial organisms before it enters the water. Such preconditioning shortens the aquatic decomposition process by allowing more rapid internal colonization by aquatic microbes and invertebrates. Decomposition is also faster in larger streams during periods of high water because the physical abrasion removes softened tissue as the wood is transported downstream or deposited on a flood plain or the outside of a bend in the stream. Only in small headwater streams or in backwaters of larger streams can rotting wood retain enough structural integrity to provide a substrate for aquatic invertebrates during the final stages of decomposition.

Dragonfly adult. (Above)
Invertebrate use of wood substrates. -Quality and texture of wood are important in determining the kinds of organisms that will colonize it. The species of wood, degree to which it is waterlogged, and decay class all affect the quality. The extent of colonization by terrestrial fungi and wood-boring insects also influences the attractiveness of the wood once it enters the water because such activity is closely associated with decay class. Dudley and Anderson (1982) found that about two-thirds of the obligate wood-using organisms were generally in soft wood; one-third in grooved, textured wood; and less than 10 percent in solid, smooth wood. In contrast, the facultative organisms occurred as follows: 20 percent on solid, smooth wood; 60 percent on grooved wood; and 20 percent on or in soft wood.
Aquatic invertebrates are also functionally classified by how they use wood substrates: (1) boring or tunneling; (2) ingesting of wood by grazing, scraping, or rasping; (3) scraping algal communities growing on wood; (4) attaching to the wood or hiding in its grooves; and (5) preying. Classification based on function are interrelated with the texture of the wood, which partly explains why a higher incidence of facultative organisms are associated with smooth, firm wood and obligate organisms with soft wood. Smooth wood surfaces are suitable for attachment and for grazing the microbial film, whereas soft wood is more easily penetrated by borers and contains fungal mycelia as a source of nutrients (Anderson and others 1978).
Page 44
Cranefly adult
Many of the surface-associated invertebrates are more opportunistic in their selections of feeding sites and habitat than are the internal associates. Invertebrates that use the surface probably do so for protection from the stream current, suspended sediments, and predation but not for obtaining food. The many grooves, crevices, and loose pieces of bark on a well-conditioned fallen tree held the majority of invertebrates collected byDudley and Anderson (1982).
Borers include some families of semiterrestrial or semiaquatic beetles (Coleoptera) and some caddisflies that hollow out twigs for cases in which they live and others that tunnel into soft wood to pupate. Fly (Diptera) larvae, however, are among the dominant borers, both in abundance and species richness. The depth to which a species (insect or microbe) can penetrate the wood is probably restricted by oxygen gradients that account for the fact that galleries are located just under the surface of the wood.
Boring activity exposes new surfaces of sound wood to microbial inoculum and colonization. When such activity is associated with some of the obligate species of fly larvae, a visible zone of stained wood often radiates outward from the larval galleries; the staining is caused by fungi that may be symbionts carried by the fly larvae. Existing galleries of terrestrial beetles increase fungal activity after the wood is submerged in water, and they are also prime habitat for cranefly (Tipulidae) larvae.i
In essence, feeding activities of borers, grazers, scrapers, and raspers result in continual utilization and decomposition of wood. The amount of wood ingested depends not only on the method of feeding and the rate of consumption but also on the firmness of the wood and the amount of fungal penetration into its superficial layer. Firm but grooved or textured wood generally has a soft, stained layer one-eighth to three-sixteenths inch (2 to 5 mm) deep that has been colonized by fungal mycelia. Grazers and shredders (beetles, caddisflies, and some stoneflies) exploit this area enriched by fungi, which contains about five times as much nitrogen as occurs in nonenriched wood.³ Scrapers (mayflies, Ephemeroptera) and raspers (snails, Mollusca) also ingest the soft layer and the periphyton-an assemblage of minute organisms attached to surfaces submerged in water.

Mayfly adult. (Above)
Many species associated with thoroughly decomposed wood are detritus feeders that just happen to be in the wood instead of in some other soft, organic material, such as leaves. The feeding and burrowing activities of the detritivores reduce the size of particles and cause mineralization of the woody material.
Net-spinning caddisflies frequently use textured, wood surfaces to attach their nets and to conceal themselves. Fallen trees also direct streamflow in a manner that provides net-spinning caddisfly larvae with ideal sites, both of surface structure and water velocity, for attaching their nets and filtering food from the water. In one study, for example, Dudley and Anderson (1982) found densities of 120 caddisfly (Hydropsyche sp.-no common name) larval nets per 12 square inches (100 cm2) of wood.
³ K. Cromack, Jr. Unpublished data on file at Oregon State University, Department of Forest Science, Corvallis, Oregon 97331.
Page 45
Stonefly adult. (Above)
Another use of wood in streams is for pupation. Many caddisfly larvae pupate on or in wood; and several families of flies, especially crane flies, bore into soft, saturated wood to pupate. In addition, many species of insects use partially submerged wood to crawl out of the water so they can emerge as terrestrial adults. Stoneflies and some species of mayflies and dragonflies exemplify taxa that seem to prefer wood to mineral substrates for emergence as terrestrial adults.
The important role of large, woody debris in creating and maintaining spawning and rearing habitat for fish has been recognized and documented within the last 10 years (Swanson and Lienkaemper 1978, Swanson and others 1976). But we are only now beginning to really appreciate the seasonal differences in function provided to salmonids by woody debris.
Summer.-Several streams were examined in western Washington so that the population biomass of salmonids in streams flowing through old-growth forests could be compared with that in recently clearcut areas (Bisson and Sedell, in press). Although the total salmonid biomass increased in a clearcut, the species richness declined to a population of steelhead trout, the majority less than 1 year old. Coho salmon and cutthroat trout, between 1 and 3 years old, were proportionately less abundant in the clearcuts. Bisson and Sedell (in press) related the shifts in composition of species and age groups to changes in the habitat caused by the cutting of old-growth trees and by the removal of large, stable, woody debris from the stream channel. The amount of stable debris declined and the amount of unstable debris increased after passage of the 1976 Washington Forest Practices Act that mandated immediate removal of debris after logging. Pool size appeared to decrease and riffle size to increase as a result of clearcutting and channel clearing. The frequency (number per kilometer or mile) of both pools and riffles appeared to decline in clearcuts, which suggested that the normal stairstep stream profiles had been altered to a more even gradient.
Pools and backwaters are used by coho salmon and large cutthroat trout; in fact, pool volume is directly related to coho biomass in coastal streams in Oregon. In addition, large, stable, woody debris is preferred as protective cover by young coho salmon, yearling steelhead, and older cutthroat trout, particularly in high-gradient river systems (Bustard and Narver 1975a, 1975b; Everest and Meehan 1981; June 1981; Lister and Genoe 1979; Nickelson and others 1979; Osborn 1981; Sedell and others 1982a, 1982b) (fig. 34).
Winter. -Most species of salmonids exhibit shifts in preference between summer and winter habitat. Large, stable, woody debris is important to the selection of winter habitat by coho salmon, steelhead trout, and cutthroat trout. The presence of large, woody debris enhances the use of different habitats within pools. Pools are preferred by all species at base streamflows during the winter. The level of preference, however, is determined by the quality of the pool and the abundance of woody debris; the more woody debris, the greater the use of the pool. Further, large, stable debris tends to attract fish more to pools along the edge of a stream than to pools in the middle of a channel.
Page 46