Growth Rates and Growth Periodicity of Tree Roots [International Review of FORESTRY RESEARCH vol 2 page181-page 236 1967]
HORST LYR AND GUNTER HOFFMANN
Institut fur Forstwissenschaften, Eberswalde, der Deutschen Akademie der Landwirtschaftswissenschaften zu Berlin (DDR)
I. Introduction - 181
II. The Root System and Growth Rates of Tree Roots. - 182
A. The Root System. - 182
B. Periodicity of Root Growth. - 194
C. Root Growth and Environmental Conditions. - 202
D. Practical Considerations 224
References. 226
I. Introduction
For a long time the root system was regarded only as an auxiliary organ of the plant providing mechanical fastening in the soil and absorbing water and mineral salts. These functions are indeed important, but it should be kept in mind that roots are highly specialized organs in which numerous syntheses are performed (Mothes, 1956). Water uptake and mineral absorption, according to modern concepts, are closely related to metabolic activity and growth of roots. From many practical experiences it is well known that vigorous root growth is necessary for good shoot growth and that disturbances in root growth impair shoot growth.
In spite of this, relatively little is known about growth behavior of roots. This is mainly because of difficulties arising from the methods used. Many failures in cultural techniques result from ignorance of the normal course of root growth and of the influences of environmental conditions upon it. Numerous questions are still almost uninvestigated,-for example, the correlative reciprocal effects between root and shoot systems, the influence of environmental factors on the intensity and course of root growth, and specific optimum values for the different tree species.
Page 181
HORST LYR AND GUNTER HOFFMANN
At present it is difficult to draw general conclusions from the statements in
the literature because environmental conditions and research techniques have
varied widely and often have been only inadequately described. As is
described in detail in the last part of this article, practical conclusions
could be drawn from better knowledge about root growth. The possibilities
in this field will be much expanded with increasing knowledge on the specific
requirements of various tree species for good root growth. Optimal
conditions for vigorous root growth are as important for high productivity as
are good conditions for shoot growth. Although this has often
been said, little is really known in detail. The physiology and ecology of root
growth are still a neglected segment of plant physiology.
II. The Root System and Growth Rates of Tree Roots
A. The Root System
1. THE GROSS ROOT SYSTEM
Many investigations on the structure of the root system of trees have been
performed during recent decades to get information on the specific peculiarities
of root morphology and on the extent of utilization of the soil volume by trees
growing on different sites. Valuable knowledge was obtained by such static
surveys on trees of different ages and on different sites. By
investigating root arrangement and distribution in the soil, practical
conclusions were drawn for silviculture and horticulture. Only some of the
most important papers on this subject can be cited here.
The root system of Fagus silvatica was studied by Zielaskowski ( 1898), Krauss
and co-workers (1934, 1935, 1939), Bonnemann (1939), Krahl-Urban (1951), Petsch
(1955), and Hausdorfer (1959). That of Pinus silvestris was investigated by
Tolsky (1904), Aaltonen (1920), Kokkonen (1923), Liese (1926), Hilf (1927),
Laitakari (1929), Wagenhoff (1938),Simanjuk (1950), Kalela (1950), Rachtejenko
(1952), Yeatman (1955), and Hausdorfer (1959). Concerning the root system of
Pseudotsuga taxifolia Britt. there are the papers of Groth (1927), Wagenknecht
(1958), and McMinn (1963); for Picea abies there are the reports of Vater
(1927), Wagenknecht and Belitz (1959), Wiedemann (1927), Krauss and co-workers
(1934, 1935, 1939), Kern et al. ( 1961 ), and Melzer (1962b). The root
system of Quercus borealis var. maxima was studied by Lemke (1955). In
addition, information on several other tree species, mostly North American, may
be found in the papers of Holch
Page 182
GROWTH OF TREE ROOTS
( 1931 ), Biswell (1934), Coile (1937), Scully (1942), Joachim (1953), Kreutzer
(1961), Dchjen (1962), and Lyford and Wilson (1964). Kreutzer (1961) and
Heikurainen (1964) have reported on root development of some tree species under
the influence of soil water conditions which were changing as a result of
amelioration measures.
The results show that it is difficult to establish general,
intraspecific rules of root development because site and soil conditions modify
root formation to such a degree that peculiarities of the species are partly or
entirely obscured (Wittich, 1947; Wagenknecht, 1955). In spite of this
limitation available knowledge of root development has allowed some conclusions
to be drawn about the practical utilization of trees in silviculture and
horticulture, conclusions which are of special importance for soils with
different layers or with high resistance to the penetration of roots
(Kvarazhelia, 1931; Wagenknecht, 1955; Kreutzer, 1961).
In many cases structure and reactivity of the root system are
of decisive significance in determining site latitude and site tolerance of a
tree species. The early development of a plant is in especially close
correlation with its ecology, because it is often decided in the seedling stage
whether a species is able to colonize a particular site. Quick reaching of
deeper soil layers which are not in danger of drying out, and a sufficient
ability to compete with roots of other species, are decisive factors in natural
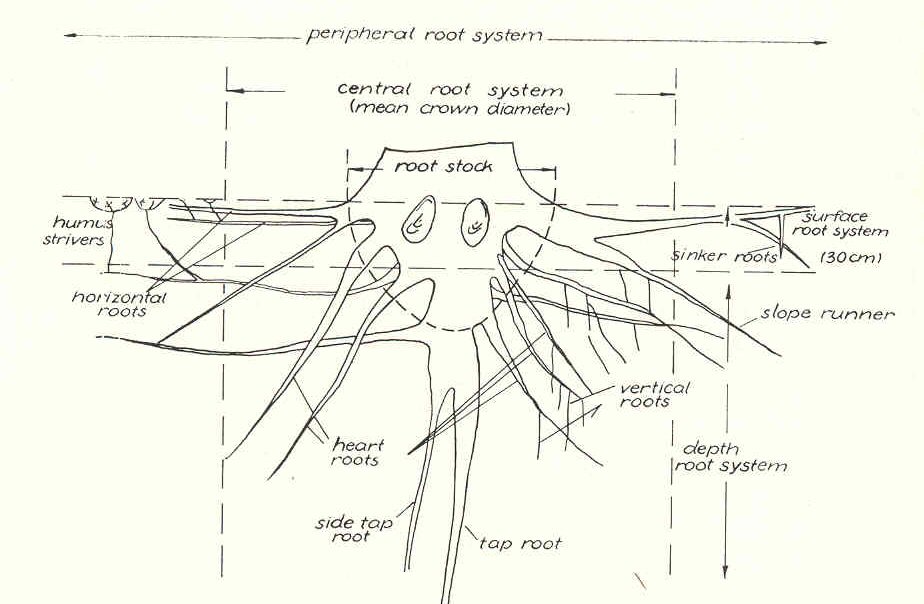
and artificial reproduction. Tree root systems consist of various types of
morphologically and functionally different roots. The most generally used
nomenclature for the different parts of the root system is given in a schematic
way in Fig. 1.
Some tree species have a tendency to form taproots. This is most obvious
in seedlings and is often found in species with large seeds rich in reserve
foods, such as Quercus, Carya, Castanea, and Juglans. In
these the taproots also serve as storage organs for the seedling. Taproots
may sometimes be observed in other genera, notably Pinus. They are well
suited for quickly reaching greater soil depths and are quickly and
preponderantly developed during the juvenile phase at the expense of the storage
material of the seeds, whereas the development of the shoots is at first rather
slow and sometimes restricted to one or very few leaves, especially so in some
desert plants (Kausch, 1959).
Taproots are able to reach low ground-water levels and secure
the water supply of a tree even in dry areas. Such roots can grow down to
considerable depths; for example, l8-year-old apple trees to 10 m (Wiggans,
1936); Robinia to 20 m (Schimper and Faber, 1935); Prosopis to 15
m; and Tamarix to as much as 30 m (Kausch, 1959). A strong horizontal
Page 183
HORST LYR AND GUNTER HOFFMANN

FIG. 1. Schematic representation of a root system with
most used nomenclature (according to Melzer, 1962b). For diameter classes
the classification of Grosskopf (1950) and Kreutzer (1961) is proposed
(approximately equivalent English terms are given here): finest roots, < 0.5 mm;
fine roots, 0.5 - 2 mm; weak roots, 2 - 5 mm; firm roots, 5 -10 mm; rough roots,
10 - 20 mm; strong roots, > 20 mm. According to Grosskopf (195O), "fine
rot capacity" means root weight or length of roots from
0.5 to 2 mm per liter of soil; "finest root capacity" means roots <0.5 mm in
diameter per liter soil.
root development may be observed on poor sites (such as sand dunes) ; where
Pinus, Betula, and Robinia may form roots 10, 20, or even 40 meters long,
which often results in considerable root competition. Roots, of Acer
rubrum may also reach a length of 25 m (Lyford and Wilson, 1964).
A shallow, superficially expanded root system, which is typical of Picea
species, is very effective in absorbing the ephemeral moisture after rains or
melting snow, especially on shallow, rocky soils, which may be an ecological
advantage in the mountains or in deserts. In the Cactaceae a
superficial root system is combined with an astonishing ability for quick
regeneration of root tips, which is a special mechanism of drought resistance
(Kausch, 1955).
In orchard trees the horizontal expansion of the root system
is normally one and a half to two times as large as the crown diameter
(Kolesnikow,
Page 184
GROWTH OF TREE ROOTS
1962b). In some cases it amounts to three to five times the crown radius (
Kvarazhelia, 1931). This seems to be true also for other trees growing as
solitaires, but the relations may be strongly modified by the environment.
This should be noted in practical fertilization of older trees, in which the
highest concentration of absorbing fine roots occurs at some distance from the
trunk. It should also be kept in mind that there exist differences in root
morphology, not only between tree species, but also between their provenances,
which may be correlated with differences in growth performance. This was
demonstrated in Pinus silvestris by Bibelriether (1964).
2. THE FINE ROOT SYSTEM
Investigations of the root system often concern only the gross root system.
Although valuable conclusions may be drawn, it should be kept in mind that the
quantity and activity of fine roots are primarily decisive for water and mineral
salt supply of a tree. At present only a few investigations have been
published in this field (e.g. Coile, 1937; Grosskopf, 1950; Grunert, 1955;
Hausdorfer, 1959; Kern et al., 1961; Buchholz and Neumann, 1964; Zottl, 1964;
see the latter for further literature).
Diameter analyses of roots of young plants show that the
major part of the root system consists of fine roots. From
Table I it may
be seen that in the four tree species investigated in detail, although only 14
to 60% of the total root weight is represented by fine roots under 1 mm in
diameter, these make up 86 to 99% of the total root length. The proportion
of the total root surface represented by fine roots is, of course, much higher
still. As may be seen from the investigations of Coile (1937) or
Hausdorfer (1959) in soil profiles, these relations are similar in mature trees,
although the weight relations may be different.
Root weight is a less suitable index than root length.
Length is better correlated with surface development, a most important. factor
affecting physiological activity and exchange of substances (Grosskopf, 1950).
With increasing soil depth the proportion of fine roots
increases. The different percentages which the single diameter classes
contribute to total root weight and length in Table I show specific
peculiarities in the root formation of the four tree species.
From our knowledge of root quantities, no direct conclusions
about root activity can be drawn, although a loose correlation may be expected
to exist. At present, detailed, comparable investigations are lacking.
Surely the activity of mycorrhizae is very important. But this activity
depends partly on the fungal species, which fact complicates the problem (Ritter
and Lyr, 1965).
Page 185
Page 186
GROWTH RATES OF FINE ROOTS
Measuring and recording the static state of the root system may be of value in solving many questions. From a physiological point of view, however, the dynamic of root development is of greater importance. Because of unequal penetration of precipitation through soil fissures, old root tubes, and differently permeable soil areas, and because of the local un-
Page 187
HORST LYR AND GUNTER HOFFMANN
equal water uptake of roots, the soil is frequently very inhomogeneously wetted.
Furthermore, the capillary water conduction of most soils is too low to equalize
the differences quickly enough, so that with increasing drying out the water
supply available to absorbing roots is essentially a stagnant one. New
water and mineral salt sources must be found by active root growth, which under
such circumstances is an important factor in ecological competition. A
high rate of root increment means the penetration of a large soil volume and the
accessibility of water reserves in the soil which is important for most trees in
maintenance of a stable water regime. Deficiency of water and nitrogen
leads to a promotion of long root growth and restriction of side-root formation.
This favors a fast penetration of the soil.
Because of technical difficulties, few attempts have been
made to measure root growth continuously. Therefore little is known
concerning maximal and average root growth rates of different trees under
similar environmental conditions. The influence of defined environmental
factors on root growth is still rather obscure. The data of different
authors diverge widely because of different methods and conditions.
In Table II some data from our own measurements and from the
literature are summarized. It can be seen that roots of fast-growing trees
may reach values which are not inferior to that for herbaceous plants.
Even the average values are considerable. It must be noted that the values
measured in the root laboratory were obtained from newly planted trees.
The disturbed root/shoot relation is normalized during the first year by
preferential root growth, and a high incremental rate is reached. The
"average" value means the average growth rate from all growing roots. This
is not the average of the whole root system, because not all roots are growing
at the same time. The data of different authors are not strictly
comparable, but the differences in methods cannot be discussed here.
4. DEPTH GROWTH
Little is known about the course of depth growth because excavations disturb
root growth, and observations in Sachs-type root boxes are possible only for a
short time and must be restricted to seedlings. In the root laboratory
(Fig. 2) at Eberswalde*1 an adequate survey on time course, periodicity, and
intensity of root growth could be obtained.
*1 Eberswalde is located at 52° 50' north latitude and 13°
49' east longitude; altitude 30 m above sea level; 572 mm annual mean
precipitation (March, 38 mm; July, 81 mm); 8.4°C annual mean temperature; 18.8°C
annual temperature variation.
Page 188
GROWTH OF TREE ROOTS

FIG. 2. View into the interior of the root laboratory at Eberswalde. Removable panels permit observations of growing roots of trees. The above-ground parts are exposed to normal outdoor conditions. Soil temperatures and other parameters can be experimentally modified.
Page 189
HORST LYR AND GUNTER HOFFMANN
rate of a tree species (see Populus,
Fig. 4).
Beyond that, specific differences exist in the rate of depth penetration.
The fast depth growth of tree seedlings with taproots has already been
mentioned. But some trees with typical heartroot, rather than taproot,
systems for example, Betula pendula or Robinia pseudoacacia-soon
reach even greater depths, which is very important for their water supply.
Strong growth during youth means more than escape from shading. In some
species deep rooting also diminishes danger from drought periods and so
increases the ability of the species to compete. In Betula papyrifera
shallow rooting on some sites leads to die back (Pommerleau and Lortie,
1962).
Figures 3 to 5 show that as growth proceeds, the center of
most active root growth (statistically represented by the solid line) shifts to
deeper soil layers. This corresponds to the normal tendency of expansion
of the root system under the influence of a specific correlative regulation.
In virgin soils deviations are to be expected because of the irregular depo-
Page 190
GROWTH OF TREE ROOTS
sition of mineral salts. Their enrichment in the upper
soil 1ayers with high humus content leads to a concentration of fine roots in
the upper horizons and thereby to a shallower rooting of most trees growing on
such soils. Under ordinary outdoor conditions as much as 80 to 90% of the
total mass of fine roots may be found in the upper soil layers (Coile, 1937;
Scully, 1942; Hausd6rfer, 1959).
In newly planted trees the tendency of the root growth center
to shift to greater depths is very common. In older trees, which have
fewer possibilities for further expansion of their root systems, other relations
are found. In these, "growth nests" are formed around some strongly
growing roots. Some roots stop growing and die; others form regeneration
roots so that a more inhomogeneous growth distribution results. This can
sometimes already be seen in the third vegetation period after planting, as, for
example, in Pinus silvestris (Fig. 6). Here root growth starts and
stops irregularly at different depths, and the density of growing roots is lower
than in younger trees (although the total amount may be larger). Kinman
(1932) made similar observations in orchard trees.
Page 191
Page 192
GROWTH OF TREE ROOTS DENSITY OF ROOT SYSTEMS
Aside from the expansion of a tree's root system, its density is probably of
great importance to the tree's ability to compete. In Figs. 3 to 5 (and in
Fig. 18 and fig. 19) distinct differences in the density of growing fine roots are
evident. Penetration of the soil is very intensive in Larix leptolepis,
Betula pendula, and Pseudotsuga taxifolia. A weak fine root
formation is characteristic of Quercus borealis var. maxima,
whereas Robinia pseudoacacia and Populus euramericana have an
intermediate position. (In Populus the very fine hair roots with a
diameter of less than 1 mm, which are typical of this species, were not
registered. If these had been included the Populus root system
would have been very much enlarged. See Table I.)
A low density may be compensated for by mycorrhizae
formation, which is not considered here. The fine fungal hyphae may
penetrate - more or less intensively, depending on the fungal species-the
between- root soil spaces and thus make accessible nearly the whole soil volume
of the root zone. However, this is of importance only in the upper soil
layers, because frequency of mycorrhizae decreases with increasing soil depth
(Preston, 1942; Werlich and Lyr, 1957). This may not depend on the humus
content and the aeration of the soil, but seems to be caused by the
physiological state of the different parts of the root system. For
example, the nodule formation in Robinia pseudoacacia grown in the open
and in the root laboratory shows very similar relations (Hoffmann, 1960; Lyr,
1963).
6. ACTIVITY IN SOIL PENETRATlON
Tree roots are forced to penetrate large soil volumes, often against the
mechanical resistance of densely packed soil layers, especially when the trees
are acting as pioneer plants. In normal stands, young tree roots may
follow old root channels formed by rotted roots or may grow in chinks of loam or
other soil crevices and fissures. From practical experience it is well
known that different tree species exhibit different activities in penetrating
compacted or dense soil horizons, which on certain sites is the decisive feature
for the choice of a tree species.
Results gained from comparable experiments on root activity,
as the ability for penetration of dense soil layers is often called, are rare.
An outstanding piece of work is that of Leibundgut et al. (1963) on the
penetration of seedling roots through artificially made compacted clay layers in
boxes. On the basis of measurements of the percentage of the total
Page 193
HORST LYE AND GUNTER HOFFMANN
root mass in the clay and in the loose layer beneath at the end of the vegetation period, the following sequence was determined: Quercus robur (26%), Alnus incana (8.7%), Alnus glutinosa (8.0%), Carpinus betulus (2.6%), Picea abies (1.6%), Pseudotsuga taxifolia (1.5%). This corresponds well to the ranking of root activity observed in the same species growing in the open. Species with a high root-to-shoot ratio seem to have a greater ability to penetrate hard soil layers. Gardner and Danielson (1964) in their experimental investigations found that optimal aeration of the soil and optimal water content of the root zone increased the ability of roots to penetrate.
7. LONGEVITY OF FINE ROOTS
Statements and opinions expressed in the literature on longevity of fine roots are very divergent. According to Kinman (1932) fine roots may die, although only days old, when the base root begins to form periderm. In other cases longevity was estimated to be a few weeks (Childers and White, 1942). Heikurainen (1955) suggested, on the basis of his studies, a longevity of roots with diameters under 1, 1 to 2, and 2 to 5 mm of 3, 5, and 10 years, respectively. In general, fine roots seem to live at least through one vegetative period, which, however, on no account is the maximal age under favorable circumstances. In the root laboratory at Eberswalde, mycorrhizae and fine roots 2 years old, and older, could be observed. The longevity of fine roots depends partly on the correlatively regulated distribution of assimilates and partly on the growth intensity of the roots of higher rank. In the open a large fraction of the fine roots may be killed periodically by drought or frost, especially in the upper soil layers, so that frequent regeneration may consequently occur. This being the situation, it is obvious that any generally valid statements on the longevity of fine roots are difficult to formulate.
B. Periodicity of Root Growth
In addition to growth rate, growth periodicity of tree roots is also of scientific and practical interest. Whereas detailed information, and in some instances reproducible results, have been obtained on shoot growth, reports on root growth are in disagreement or are contradictory. At present it is still difficult to discriminate between peculiarities of species and environmentally induced reactions. For centuries investigations of the course of root growth have been made from a practical viewpoint, but owing to difficulties with techniques conclusions have been very vague.
Page 194
GROWTH OF TREE ROOTS
Theophrastos of Lesbos (372-287 B.C.) observed that roots start growing before shoots in spring. Of the older works the following should be cited: Hales (1748), Du Hamel du Monceau (1758), von Dieskau ( 1776), G. L. Hartig (1808), Konig (1820), Lindley (1855), Th. Hartig (1863), von Mohl (1862), and Nobbe (1862). Comprehensive monographs have been compiled by Resa (1877), Wieler (1894), Engler ( 1903 ), MacDougal (1938), Ladefoged (1939), and Reed (1939).
The first contributions to our knowledge of root growth were in most cases based on casual observations during excavation or planting of trees. Systematic study of the subject was begun by Th. Hartig (1863) and Resa (1877). Hartig, specifically, gave the impulse for studies on morphological variations of the root system, and Resa for investigations on growth periodicity. Resa's method consisted in making periodic excavations of tree roots in their natural habitat to determine whether growth was or was not occurring. Similar methods were used later by Petersen (1898), Tolsky (1901), McDougall (1916), Stevens (1931), Reed (1939), Ladefoged (1939), and Vorobieva (1961).
Since the work of Stevens (1931) attempts have been made to get quantitative informations by marking the root tips. Of course only very incomplete information could be obtained by these methods because changes in soil structure and crushing and irritation of roots disturb their growth. When conclusions concerning active growth are drawn from the existence of white root tips, differences in browning of root tips introduces additional inaccuracies. Heikurainen (1955), Kalela ( 1955), and Kolesnikow (1962a ) have periodically determined root weights from soil blocks and in this way have obtained indirect data on root growth.
Busgen (1901) was the first to use root boxes for investigations on growth periodicity of forest trees. Because of size limitations only small plants can be investigated by this method. Nevertheless, it was employed later by Engler (1903), Crider (1928), Bodo (1926), Woodroof and Woodroof (1934), and L. M. Turner (1936). To permit continuous measurements of roots in their natural habitat Kinman (1932) and Rogers (1935) made trenches near orchard trees, set framed glass windows against the soil profile, and covered the pit between the measurements.
In our own investigations a root laboratory (Hoffmann 1966a) has proved to be valuable for plants from 3 to 8 years old. It consists of twelve root boxes (Fig. 2) measuring 1 X 1 m, with a depth of 2.2 m. The construction of some of the root boxes allows the introduction of measurement instruments, application of chemicals, and taking samples
Page 195
HORST LYR AND GUNTER HOFFMANN
of roots or soils. Roots with diameters of 0.5 to 1.0 mm are measured
at intervals of 1 to 2 days. Simultaneous determinations of the height
growth of the main shoots are made. According to the size of the
experimental plants, each root box contains 9 to 15 trees. Of course, only
a part of the root system is visible through the glass panels; therefore
measurements have in part been supplemented by determination of total amounts of
root masses at the end of the vegetation period. Because of temperature
differences and some influence of light, root growth in the interior of the box
may be a bit different from the visible root growth. This had already been
recognized by Engler (1903), but in the root laboratory these effects are of
little influence. When trenches are used in the open, the regeneration
arising from injured roots often gives a false picture of normal root growth
(Kinman, 1932).
In all root growth measurements "longroots" have been used
exclusively. These are the "Langwurzeln" of Busgen (1901). Such
roots have also been called "Triebwurzeln" (Bodo, 1926), "main roots" (Rogers,
1935), "growth roots" (Kolesnikow, 1962b), and "rope-like laterals" (McQuilkin,
1935). Growth measurements of roots of higher rank are extremely difficult
because of their large number, their limited growth, and their small size.
Here only determination of total weight gives reliable values.
1. GROWTH INITIATION
In general, roots of trees in temperate latitudes have a
period of rest in winter (this will be discussed in detail in the next section).
Growth is resumed in spring. The starting time depends on the tree species
and the weather. At present an exact theoretical base for a prognosis of
the initiation of root growth from climatic data is still lacking.
Richardson (1958), however, made a fairly successful attempt with Acer
saccharinum.
For a general theoretical elucidation of root rest and
growth resumption more experimental data are necessary. Probably it is a
complex process, in which hormonal relations between root and shoot are very
important, but hormonal regulation of root growth is in many aspects still
obscure (Torrey, 1956; Romberger, 1963). Furthermore, knowledge of
endogenously and exogenously influenced periods of dormancy of the individual
organs of trees is limited.
Most authors agree that root growth starts before shoot
growth, which was observed by Theophrastus 2250 years ago. Time between
root growth initiation and expansion of swelling buds is extremely variable and
de-
Page 196
G
ROWTH OF TREE ROOTSpends on environmental influences and on specific physiological optima. As detailed observations show, and the experiments of Richardson (1958) confirm, an impulse from the buds (which in this phase show a slight swelling) is necessary for root growth initiation. Apparently auxins are transported from the shoot to the root, which starts growing earlier because of a lower temperature optimum. According to Richardson (1958), roots of Acer saccharinum resume growth at 5°C, whereas bud expansion begins at 10°C. Similar differences probably exist in other tree species, but the absolute values may be expected to be different from species to species, and even from provenance to provenance. By February (in Central Europe) most trees have overcome endogenous dormancy and have entered postdormancy or the state of readiness (quiescence) in which temperature determines the time of bud opening. Increased soil temperatures lead directly or indirectly to earlier root growth, whereas bud expansion is not influenced (see Section II,C,l,a and Fig. 10).
The genus Larix is an exception to this general behavior, as was
earlier demonstrated by the data of Engler (1903) and has been confirmed by our
own measurements. In Larix species, needles of short shoots are
unfolded long before root growth begins. These new short-shoot needles
probably have the function of synthesizing the necessary assimilates for root
and shoot growth, because reserve food storage in Larix is rather
limited. Long-shoot expansion begins some time after needle unfolding and
root growth initiation (Hoffmann, 1966a).
The beginning of root growth in Quercus borealis var.
maxima is also relatively late. In Europe it may coincide with leaf
unfolding. In most trees the first root growth is made at the expense of
reserve materials. Therefore differences may exist between young seedlings
and older trees in the continuation of root growth during shoot dormancy.
Evergreen conifers, which can photosynthesize during winters, seem to behave
differently from deciduous trees. In Pinus silvestris, root growth
continues or is resumed independently of shoot growth when soil temperature is
high enough (unpublished observations).
2. GROWTH PERIODICITY DURING THE VEGETATION PERIOD
Opinions on root growth rhythm during the vegetation period are very divergent. This is not surprising, if one takes into consideration. the fact that the results were obtained from different tree species in different areas and climates and with different methods. Instead of the theoretical "normal distribution" or bell curve of mass increment, root growth as well as shoot growth has a very irregular time course (Figs. 7 and 8).
Page 197
HORST LYR AND GUNTER HOFFMANN
GROWTH OF TREE ROOTS
Resa (1877) assumed an antagonistic interrelation between root growth and
shoot growth. This means that in months with strong shoot growth only a
limited root growth or none at all should take place, and vice versa. In
contradiction to this theory, Wieler (1894) and Busgen (1901) pointed to a
functional relation between root and shoot and argued that strong root growth
must be bound to strong shoot growth. On the basis of comprehensive
measurements Engler (1903) came to the opinion that in Central Europe all tree
species have a maximum period of root growth in May and June, which is
interrupted in August by a rest period, followed by a second but lower peak in
autumn. Such a diminution or interruption of root growth in midsummer has
often been described (McDougall, 1916; L. M. Turner, 1936; Kolesnikow, 1962b).
But other authors (Tolsky, 1901; Hesselink, 1926; Rogers, 1935; Roze, 1937;
Reed, 1939; Ladefoged, 1939) found no typical growth curves with two distinct
peaks, which agrees with our own investigations.
We regard midsummer root growth cessation as due to
unfavorable environmental conditions (periods of drought or high temperature).
Under equivalent environmental conditions the rhythm of root and shoot growth
differs from species to species and even from one individual tree to another.
Some comparable curves for several tree species are summarized in Fig. 8.
Because of the changing weather conditions, growth curves for different years
are quite divergent, and generalizations are not yet possible. In our own
measurements, neither significant antagonistic nor synergistic interrelations
between shoot and root growth could be found by mathematical analysis.
Interpretation is further complicated by the fact that at the same time growing
and nongrowing roots may be found (Stevens, 1931; Ladefoged, 1939; Wilcox,
1954), so that their proportion should be determined. The correlation
between environmental conditions and root growth is probably very complex,
because besides direct influences of soil factors many indirect influences may
act upon roots via primary effects upon activity.
Only the most general rules of root growth in a moderate climate can be stated here. Maximal root growth, with regard to both the number of growing roots and the total growth in length, in most tree species occurs in the early summer (June and July) (Fig. 8). Seedlings with early termination of shoot growth (Quercus type) often exhibited strong root growth in midsummer. Regarding the growth rate of individual roots, a maximum in early summer is evident, especially in deciduous trees (Populus, Robinia, Quercus, etc.), whereas conifers (Pinus silvestris, Picea abies, Larix decidua, and Pseudotsuga taxifolia) show a more uni-
Page 199
form growth throughout the whole vegetation period, (Fig. 8). During
August, but especially in September, root growth begins to diminish (Hoffmann,
1966a). Shoot growth in most species has stopped by the end of August or
early September, often much earlier. Towards the end of the vegetation
period the number of growing roots decreases considerably, and longer pauses in
growth of individual roots can be observed. The measured values are often
represented by only a very few roots.
Some species show peculiarities in root growth. For
example, in Pinus silvestris root growth is very weak during the time of
formation of new shoots and needles and increases considerably after expansion
of the
Page 200
GROWTH OF TREE ROOTS
needles. This may be true also in other Pinus species; it seems to be correlated with the strong consumption of assimilates by the growing shoot. During this growth phase negative balances of assimilation have been measured (Neuwirth, 1959). .
3. TERMINATION OF ROOT GROWTH
In areas having low winter temperatures, root growth usually stops in the
autumn. In the climates of Eberswalde, root growth of most trees ceases in
September or October; only in some years does growth continue into November or
even December. This was observed in 1963 in Betula pendula and
Pinus silvestris. In all species root growth continues longer than
shoot growth and can go on after leaf abscission. This is remarkable,
because at this time (end of September or beginning of October) shoots are often
already in deep dormancy (Vogl and Kemmer, 1961). Evidently there exists a
certain autonomy of root growth, and it may be that- contrary to the condition
in shoots-no internally controlled period of dormancy is present in roots.
This is confirmed by observations that artificial heating of the soil extends
root growth considerably (see Fig. 10) and that keeping of trees (Pinus
strobus) in a warm greenhouse leads to continuous root growth (Stevens,
1931).
Several authors have described a winter growth of roots.
This seems to be restricted to regions with mild winter temperatures and
frost-free soils. It was observed for conifers and deciduous trees in the
southern part of the United States, in British Columbia, in the Crimea, and in
parts of Europe (Du Hamel du Monceau, 1758; Harris, 1926; Crider, 1928; L. M.
Turner, 1936; Kolesnikow, 1962b). Apparently both evergreen trees and
deciduous trees may have an uninterrupted root growth under certain
circumstances. But in all cases a diminution of growth rate and the number
of growing roots during the winter period has been reported.
4. DIURNAL GROWTH RHYTHM
Very few data exist on diurnal growth rhythms of roots. Kolesnikow ( 1962a) mentioned that growth should be stronger at night than during the day. Exact measurements in the root laboratory at Eberswalde have confirmed these statements. Although active root growth occurs during both day and night, it is more rapid at night. With day growth of a species taken as 100%, night growth of the same species gave on an average the following relative values: Populus trichocarpa 160; Quercus borealis var. maxima 137; Pinus silvestris 136; Picea abies 130. The diurnal rhythm of shoot growth is much more variable. Whether diurnal
Page 201
HORST LYR AND GUNTER HOFFMANN
growth rhythms are caused by internal periodicity or only by the externally regulated periodicity of photosynthesis, translocation, and transpiration has not yet been investigated.
C. Root Growth and Environmental Conditions
1. ROOT GROWTH AND SOIL TEMPERATURE
Because root growth, as well as mineral salt and water
uptake, is dependent on metabolic processes, it is to be expected that soil
temperature will influence root growth and activity and indirectly whole plant
growth also. Shoots are dependent on roots for a sufficient supply of
water, minerals, and some organic compounds. According to Tew et al.
(1963), soil temperature has an even greater influence on transpiration than
have air temperature and humidity.
Investigations on the effect of soil temperature on root
growth are complicated by the fact that growth intensity of roots depends not
only on temperature-as it might in heterotrophic microorganisms on an optimal
medium, or in isolated roots in artificial culture-but also on soil moisture and
shoot activity (carbohydrate supply), which are themselves influenced by light,
air temperature and humidity, and root activity. Therefore no simple
dependence of root growth rate on soil temperature can be expected under natural
conditions. As our own measurements show, the rate of root growth during
the vegetation period changes much more than soil temperature. The latter
is a dominant factor only in spring and fall, because it acts together with
other factors, such as soil moisture and shoot activity, during the summer.
Most authors agree that optimal temperatures for roots are lower than for shoots
of the same species. This is demonstrated by the experiments of Richardson
(1958), for example.
a. Cardinal Temperature Values. It is difficult
to give useful values for minimum, optimum, and maximum temperatures for root
growth of trees. Most authors have not distinguished between a
physiological and an ecological optimum and have neglected the influence of
other factors on these cardinal values. The method of measuring growth is
very important in determining the temperature values. Therefore most data
are not strictly comparable. Short-term measurements of root growth rates
(Ladefoged, 1939) reveal the physiological optimum temperature (Fig. 9), which
can also be obtained with isolated cultured roots. Ecological optimal
values, on the other hand, are dependent on the carbohydrate balance and other
factors. A long period of higher soil temperature can
Page 202
GROWTH OF TREE ROOTS
lead to a negative carbohydrate balance because of enhanced root respiration.
In many cases the ecological optimum, therefore, lies below the physiological
optimum. In Table III some cardinal temperature values from the literature
are summarized. It can be seen from these data that the range of
temperature in which growth is possible lies between +2° and +35°C.
Distinct differences in cardinal temperature values exist
between species. This is probably true for provenances also.
According to Aaltonen (1942), Pinus silvestris is more thermophilic in
its root growth than is Picea abies.
The most exact data are those concerning the minimum
temperature, because its determination from growth initiation or growth
cessation is relatively simple. But even here the physiological and
ecological values may be different. Picea abies, Abies alba, Fagus
silvatica, and Acer pseudoplatanus evidently have a rather low
minimum. Growth begins or stops in the vicinity of 2° to 4°C. On the
other hand, Citrus species
Page 203
HORST LYR AND GUNTER HOFFMANN
begin root growth only when the temperature rises above 11°C (Muromtsew,
1962). Roots of some Malus varieties and Prunus persica may even
be called thermophilic.
A comparison of optimum temperatures from the literature is
at present nearly impossible because physiological and ecological values are
confused and the definition and determination of an ecological optimum are still
obscure. Physiological data give information on the temperature at which
the highest growth rate of roots has been observed, but it does
Page 204
GROWTH OF TREE ROOTS
not follow that heating the soil to such a temperature would give an optimal
effect on an ecological time scale. Here further investigations are
needed.
It is striking that most physiologically
optimal values lie above 20°C, temperatures which only rarely are reached in the
soil. Although the ecological optimum may be lower than the physiological, it
must be expected that on many sites soil temperature is suboptimal. In
some cases it can even be the limiting factor. In our
own experiments with Robinia pseudoacacia, artificial soil heating
(increase of temperature about 5°C
above normal) induced root growth which began 41 days earlier and terminated 44 days later than in control plants, whereas shoot growth (normal climate in the open) began only one day earlier (Fig. 10). The growth rhythm of the shoots in the two groups was similar, but the final height of shoots with soil heating was increased by 15% (11 cm). This effect seems to have been caused by the enhanced metabolic activity of the roots. Because R. pseudoacacia is a thermophilic tree species, further experiments are needed to show whether this is true for other species also.
Page 205
In nurseries, soil mulching with plastic film can be used to increase soil
temperature and conserve moisture. Sorgum vulgare showed an in-
crease In yield of 68 to 76% after such treatment (Pusztai, 1963).
However, no such data for tree species are yet available.
The effect of temperature on root growth is complicated by
interrelations of root and shoot temperatures. Low root temperatures and
high shoot temperatures favor shoot growth, and the inverse also holds, but high
root or shoot temperatures can counterbalance low root or shoot temperatures to
some extent (Hellmers, 1963).
The maximum temperatures are of practical importance only in
special cases. In hot and dry regions (semidesert afforestations) root
growth may be limited by high temperatures. This may also happen in
temperate latitudes during dry periods in the season of strongest insolation.
But here only shallow roots are directly influenced, and the danger of drought
is more serious than that of heat.
Muromtsew (1962) pointed out the fact that plant species have
a different amplitude of temperature for root growth. Citrus, for
instance, belongs to a group with a small amplitude (7°C), whereas strawberries
have a wider one (16°C). This seems to be related to the normal climatic
temperature amplitude of the indigenous region. Therefore it is to be expected
that trees from the tropics and subtropics should have a narrow amplitude, and
trees from temperate and cold regions a progressively wider amplitude. At
present, exact and comparable values are lacking. But it seems possible
that site tolerance of a species can be limited by the soil temperature, which
is especially important in the cultivation of exotic trees. Insufficient
root activity as a consequence of low soil temperatures could be a reason for
the natural tree line in the alpine and northern regions, because plants suffer
from desiccation due to high transpiration and limited water uptake (Michaelis,
1934).
It should be mentioned that temperatures induces
morphogenetic changes in roots. Isolated roots of Robinia show an
inhibition of lateral root formation at 19°C and an inhibition of length growth
of the main root at 33°C, which favors side-root development (Seeliger, 1959).
Similar results were obtained by Slankis (1949) with Pinus silvestris.
Nightingale (1935) observed that roots of peaches were glistening white with
large-diameter, succulent, and fragile tips when grown at 24°C. Roots of
Sequoia seedlings appeared to be the healthiest, but not the longest, at
18°C. They were short and thick at 8°C, and thin with fewer and shorter
white root tips at 28°C (Hellmers, 1963).
Page 206
GROWTH OF TREE ROOTS
2. ROOT GROWTH-SOIL MOISTURE AND SOIL AERATION
Besides soil temperature, soil moisture influences root growth considerably. Useful growth measurements are complicated by the fact that the water uptake by an individual root does not determine its growth rate. A sufficient water uptake by a part of the root system can provide the necessary water for the whole system. Therefore some roots of a system can grow through dry zones when an internal water supply is guaranteed by water uptake of other roots (Shautz, 1927; Kausch, 1959). Because of the unequal distribution of moisture in the soil, this fact is of considerable ecological significance. Of course, mineral salt absorption is inhibited in dry soil zones, as was demonstrated by Hunter and Kelley (1946) with P
32.Page 207
HORST LYR AND GUNTER HOFFMANN
content (Buchholz and Neumann, 1964). On the other hand, roots avoid areas of excessively wet soil (Howard, 1925). Roots in swamps lie near the surface, and root growth in depth depends on changes in the groundwater level (Busarova, 1961).
In dry soils the root system has a higher portion of the total plant weight. With increasing soil moisture content the root portion decreases in favor of the above-ground organs (Tolsky, 1904; Aaltonen, 1920; Huber, 1924; Rogers and Vyvyan, 1928; Volk, 1934). In drier soils not only is a larger soil volume made accessible for water absorption by an extensive
root system, but the chances of roots encountering scattered local areas of
higher water content are also increased. The root system can begin growth
anew during drought periods if parts of the system are wetted, thereby
increasing the internal water supply to all the roots {Bormann, 1957; Kausch,
1959). Water deficiency leads to an inhibition of root growth before
cessation of shoot growth or any visible injury becomes evident (Rogers, 1935;
L. M. Turner, 1936; Ladefoged, 1939; Leyton and Rousseau, 1958; Kokhno, 1959).
Figure 12 shows this behavior for Larix leptolepis in Germany during the
drought of 1964.
Root suberization is accelerated in dry soil and the
effective absorbing surface is thereby diminished, so that root systems do not
regain their full capacity for water uptake rewetting until some regeneration of
Page 208
GROWTH OF TREE ROOTS
growing tips has occurred (Kramer, 1950). When the soil becomes very dry, parts of the root system may die. This is common in surface soil layers (Buchholz and Neumann, 1964). Therefore water and mineral salt uptake remains diminished even for a long time after conditions return to normal following a severe drought. This, in turn, retards root regeneration by inhibition of photosynthesis and brings long-lasting growth depressions in older stands.
b. Water Surplus. Some sites suffer permanently (swamps and peat bogs) or intermittently (Hood plains) from an excess of water in the soil. Water excess-especially in connection with slow water movement- implies a low oxygen availability in the soil. As the results of artificial water cultures demonstrate, tree roots are in general not sensitive to water saturation of the medium, provided that sufficient aeration is main-
Page 209
HORST LYR AND GUNTER HOFFMANN
tained. However, ordinarily water saturation of the soil results in a deficiency of oxygen and an enrichment of carbon dioxide, which shifts the redox potential. Furthermore, wet soils are apt to be cold. This lowers the mineral salt and water uptake (Kramer and Kozlowski, 1960); therefore, Hooding or raising of the ground-water level inhibits growth or induces dieback in susceptible tree species as an indirect consequence of an insufficient root activity. Symptoms often first appear during a subsequent drought period, when water supply to the shoot by the partly dead or injured root system is inadequate. Dieback of tree stands in large areas may set in when, because of oxygen deficiency, reduced compounds are formed in the soil which poison the roots (H
2S from peat, for example) (Trenel, 1932). On peat bogs, as well as on frozen soils, severe symptoms of mineral salt deficiency are common, although often mineral salt content of the soil is not particularly low.Page 210
GROWTH OF TREE ROOTS
reduced at 15% O
2; at 3 to 5% poor growth still can be observed; and at 0.1 to 3% the minimum is reached (Boynton, 1940; Boynton and Reuther, 1938). Only a few species can grow when available oxygen falls below 2% of the soil atmosphere (Fig 13). In the absence of oxygen, roots die after some time. Growth periodicity is of importance in so far as the oxygen requirement during dormant or inactive periods is lower and tree roots are less sensitive. Salix, Alnus, Betula, and some other genera probably are able to provide their roots with some oxygen through an intercellular space system, enabling these species better to tolerate sites poor in soil oxygen (Huikari, 1954; Leyton and Rousseau, 1958). This principle is extremely effective in water and swamp plants which have a large intercellular space system (aerenchyma). In the environs of roots with an internal oxygen supply, heavy metal sulfides in the soil are reoxidized.Page 211
HORST LYR AND GUNTER HOFFMANN
3. ROOT GROWTH AND MINERAL NUTRITION
Mineral nutrition also influences root growth and root morphology.
In spite of numerous accounts in the literature, it is seldom clear whether the
effects described are unspecific and resulting from general promotion of plant
growth (for example, as a consequence of fertilization), or whether there are
specific influences of mineral nutrition on root growth {Mengel, 1965). The
latter have been indicated in some instances, but in general it must be observed
that mineral nutrition is closely related to other growth factors such as water
supply and shoot activity, and effects of mineral nutrition per se are difficult
to ascertain.
It is well known that root development in poor soils is
comparatively stronger than in rich ones (Schwarz, 1892; Busgen, 1901; Rogers
and Vyvyan, 1928). The root-to-shoot ratio may be nearly 1 : 1 in poor
soils, whereas it decreases in better soils to about 1 : 2. Zottl (1964),
therefore, with only moderate simplification, states that in vigorously growing
stands increased increments of stem wood after fertilization are obtained with
unstimulated or only slightly increased root systems. On the other hand,
in nutritionally poor stands, additional growth of above-ground parts requires a
strong enlargement of the root systems.
A striking fact is the concentration of fine roots in nutrient-rich zones of the
soil. Thus stimulated root growth in soil layers rich in humus is often
recorded in the field (Moller, 1903; Albert, 1928; Wagenknecht, 1941; Grunert,
1955; Hausdorfer, 1959). In part this may be a nitrogen effect, as it is
particularly evident in soils poor in nitrogen (Ehwald et al., 1963). But
evidently other nutrients also act in a similar way, because the same phenomenon
can be observed in layers of coarse sand with high contents of silicates and in
strata of clay or heavy minerals (Fig. 14). A direct influence of soil
moisture, although sometimes strengthening the effect, may be excluded.
This behavior is caused by the general reaction norm of the root system, which
in a state of nutrient deficiency primarily forms poorly branched long roots
("seeking or "pioneer" roots).
In soil layers rich in nutrients, the growth of the main root
is lessened at the expense of a strengthened development of side roots,
resulting in dense root development in these layers (Fig. 15). This
antagonistic behavior between length growth and side-root development is a
characteristic feature (Kausch, 1959) which results in ecologically useful
modifications of root systems under the influence of certain site factors.
Furthermore, it explains the observations that the rooting quotient {total root
length/number of root tips) of orchard trees is very high in sandy soils
Page 212
GROWTH OF TREE ROOTS
and is reduced after fertilization (Otto, 1964). Various clones,
however, may react somewhat differently in this respect. Lundegardh (1957)
suggested that the favored length growth induced by nitrogen deficiency be
called "nitrogenium-deficiency-etiolement."
In addition to the above, it must be noted that the mineral
nutrient status (and especially nitrogen supply) affects the root-to-shoot
ratio. Therefore, in the field, differences occur in intensity of rooting
in various humus forms with different nitrogen contents (Table IV). In raw humus
Page 213
HORST LYR AND GUNTER HOFFMANN
poor in nitrogen, Fagus silvatica forms about twice as much length and weight of roots per square centimeter of leaf area as in mull (Meyer, 1963).
Humic acids and quinoid compounds have been reported to elicit specific plant growth effects. Stimulation of root growth has also been
reported (Flaig, 1958; Giulimondi, 1961). Little is known about the
possible effects of other biogenic compounds formed in the soil on root growth
and development.
Specific effects of different nitrogen compounds are
noteworthy.
Page 214
GROWTH OF TREE ROOTS
Leyton (1952), Prjanischnikow (1952), and Smith (1957) found that the root
systems of plants supplied only with nitrate are stronger than of those plants
fertilized with ammoniacal nitrogen. Evers (1964) also considers nitrate
to be the best form of nitrogen for growth of poplar. Alnus glutinosa
fertilized with nitrate develops very dense, fine, and abundantly branched
root systems, whereas ammoniacal fertilization causes formation of long and
sparingly branched roots. Under field conditions there is probably a
relation between soil aeration and the form of soil nitrogen.
An extensive and rather contradictory literature exists
concerning the effects of other elements on root growth and the formation of
root
systems. Because of ion antagonism and multifactorial effects which may lead to variable results in different cases, valid generalizations for woody plants are not yet possible. (For further literature see Kramer, 1956.) Fertilization of forests generally stimulates superficial rooting because vertical translocation (especially of phosphorus) is rather slow. Furthermore, the humus content and the recycling of nutrients by soil organisms favor the enrichment of nutritive elements in the upper soil horizons. Buchholz and Neumann (1964) found that in a 56-year-old pine stand the superficial rooting was doubled two years after nitrogen fertilization, while at the same time the deep rooting decreased (Fig. 16).
Page 215
Click here for Table V Page 216-217
HORST LYR AND GUNTER HOFFMAN
4. ROOT GROWTH AND LIGHT INFLUENCE
The formation of the root system is dependent-as is plant growth as a whole-on the photosynthetic efficiency of the tree, which means that root growth is in competition with shoot growth for carbohydrates. In higher plants a general and ecologically useful reaction norm in the distribution of carbohydrates has evolved, especially when shading makes
carbohydrates limiting. Increasing shade decreases growth as a whole but
leads to a relative stimulation of shoot growth at the expense of root development (Fig. 17,
Table V) (Mitchell, 1936; Gast, 1937; Kozlowski, 1949; Lyr
et al., 1963). In short, shading primarily influences root growth, and the
root-to-shoot ratios are thereby altered (Table VI).
The intensity of the effect, however, varies according to the shade tolerance
of the species involved. From this it is clear that at reduced light intensities
the ability to wage root competition decreases in shade- intolerant species. This
effect may be reinforced by other factors that
Page 218
Page 219
HORST LYR AND GUNTER HOFFMANN
induce a relative reduction of root growth-for example, nitrogen fertilization (Lyr et al., 1967). Shaded plants are therefore apt to be more susceptible to drought than others in full light (Kramer and Decker, 1944; Barney, 1951). At a light reduction to 40% of daylight, which is by no means an extreme shading degree under field conditions, both rooting depth and rooting density of Robinia are strongly reduced (Figs. 18 and
19). Whereas plants in full daylight showed a total length of fine
roots of 266 m, the corresponding value in the shaded ones was only 39 m
(Hoffmann, 1965). In Lupinus and Alnus, shading greatly
reduces nitrogen fixation (Hoffmann, 1960; Lyr et al., 1963). Some
other tree species show remarkable reduction in formation of mycorrhizae when
shaded (Bjorkman, 1942).
W. Turner (1922) and Shirley (1929) earlier pointed out that
the strongest root systems are developed in full daylight. The former
believed that surplus quantities of assimilates not required for shoot growth
are used for root growth. This may be only partly correct, however.
If a
Page 220
GROWTH OF TREE ROOTS
sufficient nitrogen supply is provided, shoots compete with roots whenever environmental conditions allow growth. Whether trees of the Populus type, having long duration of shoot growth, and of the Quercus type, which have a short growth period (Lyr and Hoffmann, 1965), behave differently in root/shoot competition has not yet been determined.
Reduction of photosynthetic activity, although caused by shading of the leaves, results in decreasing root growth, but not immediately. The reaction of the roots takes place after 12 to 24 hours in Acer saccharinum (Richardson, 1953a,b). This may be the reason why root growth is more active at night than during the day (Section 1I,B,4). In young Acer seedlings this root reaction is more rapid, whereas in Quercus the reaction of root growth as a consequence of shading takes place more slowly, presumably because of higher food reserves in this genus (Richardson, 1956). The negative phototropism of roots is well known. But various species are likely to exhibit different sensitivity. Isolated roots of some tree species show decreased growth in the light (Seeliger, 1959).
Page 221
HORST LYR AND GUNTER HOFFMANN
5. ROOT/SHOOT INTERRELATION
Root growth and shoot growth are closely interrelated. Physiological
regulation mechanisms of an unknown nature provide for a balanced root-to-shoot
ratio adapted to the ecological conditions. Probably hormonal mechanisms
which determine correlative food distribution are of great importance.
There exist some generally applicable reactions norms, which vary quantitatively
from species to species. The root as a heterotrophic organ is dependent on
the shoot for a supply or carbohydrate and some vitamins. This can be
demonstrated with cultures of isolated roots (Slankis, 1949; Seeliger, 1956,
1959; Ulrich, 1962). The level of food and auxin supply from the shoot to
the root depends on the conditions affecting photosynthesis, on leaf age and
leaf area, and on the utilization of photosynthate within the shoot. When the
later is low-for example, in rooted leaves-a large root system is built up very
soon. The reaction of root growth to changes in rate of photosynthesis and
respiration is, therefore, understandable (Richardson, 1953a,b; see also above).
Although roots are not dependent on auxin supply from shoots,
hormone production of shoots seems to influence root development in a specific
manner. According to Richardson (1958) defoliation inhibits length growth
of roots but not root-sucker formation. Defoliation of the leading shoot
bud inhibits formation of new roots, whereas roots already present go on
growing. f3-lndoleacetic acid can counteract the effect of decapitation.
Probably still other hormones participate in the regulation of root growth
(Romberger, 1963).
Environmental factors and cultural practices can shift the
root -to - shoot ratio by incompletely known mechanisms. Soil dryness,
mineral salt deficiency (especially of nitrogen), and higher soil temperatures
cause an increase in ratio, whereas shading, nitrogen fertilization, higher air
temperatures, and sufficient soil moisture induce a decrease in the
root-to-shoot ratio. Pruning, crown cutting, and defoliation act like
shading and inhibit the development of the root systems (Chandler, 1923;
Heinicke, 1936; Sawage and Cowart, 1942; Wood, 1939; Hoffmann, 1966c).
Mowing and grazing of herbaceous plants decreases their root
formation and the ability of their roots to compete (Weaver and Darland, 1949;
Kohnlein and Vetter, 1953). Development of the root system may also be
reduced by heavy fruiting. Chandler (1923) found a 50% decrease in the
root system of Prunus after a heavy crop, and, according to Nutman
(1933), Coffea arabica can be injured or killed by high harvests because
of the insufficient supply of carbohydrates to the root system.
Page 222
GROWTH OF TREE ROOTS
Some data indicate that typical species-to-species
differences exist in the root-to-shoot ratio. This has been investigated
mostly in young trees (see Table VI).
According to Lobanow (1960), the ratio of root surface to
leaf surface is less than 1 in strongly mycotrophic trees (such as Pinus,
Picea, Larix), whereas nonmycotrophic species reach much higher values.
A value of 139 has been reported for rye (Dittmer, 1937). Mycorrhizae
probably influence the root-to-shoot ratio by providing additional absorbing
organs.
In older trees it is assumed that about 20% of the total
weight is roots (Rogers and Vyvyan, 1934; Ehwald, 1957; Assmann, 1961).
According to Bray (1963) and Whittaker et al. (1963), root-to-shoot
ratios decrease with increasing age of trees. The above-ground and
subterranean development of a tree are always in close correlation. The
more exactly the real assimilation efficiency and the root quantity are
ascertained, the better can this correlation be determined. The crown
radius, for example, gives a lower correlation than the crown mantle area
(Melzer, 1962a).
The root-to-shoot ratio changes during the growing season
because of the somewhat independent development of roots and shoots. This
is evident in Fig. 8. Trees having a
Quercus type of growth flush show this most distinctly. A typical
course for Picea glauca (Mullin, 1963) is shown in Fig. 20.
Finally we want to mention some peculiarities.
According to Lemke ( 1955), clear correlations exist in Quercus borealis
var. maxima between crown size and trunk diameter on the one hand and the
mean root depth and range of horizontal roots on the other. Large crowned
trees have a denser root systems than others. In contrast to other trees,
the horizontal roots of Pseudotsuga taxifolia reportedly do not reach
beyond the crown projection area (Wagenknecht, 1958), which is important from
the silvicultural viewpoint. In this same species a one-sided crown
development is associated with a one-sided formation of the root system.
The management of stands and trees for good crown formation
is a possible way to assure the production of large, deep-reaching root systems
which give a sure protection against windthrow (Wagenknecht and Belitz, 1959).
Whereas in general dominant trees develop a deeper root system than suppressed
ones, in Fagus silvatica root depth is quite independent of the socia-ecological
position of the tree (Wagenknecht, 1960).
It is interesting that the formation of a new leading shoot
from a flat-topped crown in Pinus silvestris is correlated with the
regeneration of a new taproot (Albert, 1907; Wagenknecht, 1960). According to
Page 223
HORST LYR AND GUNTER HOFFMANN
Romer and Hilkenbaumer (1936, 1937) scions also have a specific influence on growth rate and branching of roots of orchard trees. Similar observations were made by Kemmer (1964).
D. Practical Considerations
In efforts to increase output of forest products by
application of a highly developed forest science, there is a need for exact
knowledge of the causal connections between environmental effective factors and
tree response. In silvicultural decisions, knowledge of root growth and
the factors influencing it should play a more important role than it has in the
past.
It is possible, by numerous practical steps, to influence
root growth and development of the root system in order to increase tree growth
and to protect forests against harmful biotic and abiotic agents. There
are data indicating that it is likewise possible by breeding to select clones or
populations of trees which have the desired qualities with respect to root
formation and root activity.
Some management measures based on knowledge of root behavior
are already being applied in the forest. It is well known, for instance,
that crown-tending diminishes the danger of wind throw on wet or shallow soils
where trees have a tendency to form superficial root systems.
Page 224
GROWTH OF TREE ROOTS
There is a fund of practical experience concerning the
suitability of various tree species for heavy, difficulty penetrable soils, such
as pseudogleys. On such soils, as well as on periodically flooded sites, a
high root activity and a low demand for exogenous oxygen are decisive factors in
determining site tolerance of a tree species.
Tree species in mixtures that have proved to be highly
productive are often complementary to one another in the utilization of the site
by the root system as well as in utilization of light. Therefore mixed
stands often are more productive than pure stands (Erteld, 1953). New
mixtures and mixture ratios can be developed theoretically from the knowledge of
the general physiology of the trees and of the behavior of their root systems.
This is very important in the tropics, where root competition is often a
dominating factor.
Soil temperature has often been left out of consideration as
a site factor, although it has been demonstrated in several instances that an
increase of soil temperature brings an increase of yield. The failure of
some trees on cold soils is caused by an insufficient root metabolism which
causes deleterious effects because of an inadequate supply of water, mineral
salts, and essential organic compounds to the shoot. The periodicity of
root and shoot growth as well as the varying ability to regenerate roots during
the vegetation period should be considered in the choice of planting times and
times of tending and fertilizing young plantations.
Preparation of the soil should be done in such a manner that
sufficient aeration is guaranteed and a deep-reaching, drought-resistant root
system is formed. This may be achieved on some soils by a suitable
layering of the humus by plowing.
Fertilization should be done in such a way that a harmonious
root-to- shoot ratio is maintained. This is important in nurseries to
minimize losses arising from climatic anomalies. Development of a shallow
root system as a consequence of fertilization should be counterbalanced by other
measures specifically intended to promote deep rooting.
Light conditions in stands can be influenced so that strong
root systems are developed which have a high ability for competition and for
utilization of deeply situated water and mineral salt sources. Here
differences between tree species should be observed and considered in selecting
species combinations in mixed stands.
Many failures of cultural techniques as well as growth
stagnation of trees and stands have their cause in poor root growth or in
damages to the root system. Their early recognition and amelioration are
important
Page 225
HORST LYR AND GUNTER HOFFMANN
factors for increasing production. The rate of regeneration of the root
system, which can be favored by several practical measures, often determines the
duration and severity of growth interruptions and losses of increment.
Further examples of possibilities of increasing wood
production by practical application of knowledge about root growth and root
behavior could be given. At present, unfortunately, detailed data on
quantitative differences in the behavior of tree species and their provenances
are still lacking. Available observations are too sporadic to permit valid
conclusions to be drawn from them. Therefore, reviewing the literature and
summarizing the facts and opinions found therein is not easy. But in our
endeavors we should not shrink from attempting to analyze the complicated
physiological processes of interaction with the environment and from striving to
build up a theoretical base for a high silvicultural production.
Page 226
REFERENCES
If you live in USA I can fax references to you. Here is the option to download.
To save these files to your PC, right click on the hyperlink below and select the menu option "Save Target As...". Select the folder on your PC where you would like the documents to be saved and hit the "Save" button. PFD Files. (Est. 10mg)