
[MA149]Pages 117-145
(It may take a while to download but I think it is
worthy - John A. Keslick, Jr.) A word from the webmaster - Within
this article the word nutrient is misused at times where the true meaning is
essential elements. See my dictionary at
www.treedictionary.com and look up
nutrient as well as essential element. The reason for the latter is to
reduce misunderstanding of terms to better understand the message.
John A. Keslick, Jr.
Depletion of finite resources
As the "Grand Exploiters," we now face a world soon to encounter shortages of
its available minerals, metals, fossil fuels, and - if we do not act now -
agricultural lands.
Agricultural land, forests, open space
Linked closely to the manipulation of the environment is the constant depletion
of our most valuable finite resource -land. It is necessary, of course, to
change land uses as our population increases, but we must do this wisely.
Each year, in the name of "progress," we pave over, build on, or otherwise
remove forever thousands of acres of productive land. As was noted
earlier, some activities- building on flood plains and filling of inland
wetlands and salt marshes - produce adverse effects that are complex and
far-reaching.
The removal of productive farms and forests, now proceeding
rapidly in highly populated areas of the United States (fig. 46), may directly
affect our well-being. The current trend of concentrating food production
in limited geographic areas that are often far from population centers may prove
unsound as transportation and storage costs increase and as regional crops fail
due to climatic disasters or disease.
How much land is needed for agriculture, forestry, or
protective open space? How should we ensure that sufficient amounts be
maintained? These questions are extremely difficult to answer. It is
often not recognized that much more space is demanded by each person in an
affluent nation such as ours than is needed in poorer nations. This is due
to the foods we desire, the fiber we use, and the human services we demand to
maintain our standard of living.
Some ecologists have recommended that at least one-third of
all land be maintained as open space. Whether or not this figure is the
goal we should strive for, the current rate of land use changes and the
projected doubling of our population in 25 to 45 years make it imperative that
means be developed to ensure that the destruction of our productive agricultural
lands is halted.
Page 117
Figure 47. - If rates of exploitation and "throwaway" practices continue to increase, the known reserves of nearly every essential metal and mineral may be depleted
by 2100.
Page 118
Minerals and metals
We in the United States have used more minerals and
fossil fuels in the last three decades than have been used by humanity since
time began! Our mineral resources are running out (fig. 47).
If exploitation increases at its present rate, and if we continue our
"throwaway" practices, those of us still alive in the year 2000 may see the last
of the copper mines close-and before them, the last lead, tin, zinc, silver,
mercury, and gold mines. And the reserves of most of the other essential
minerals and metals possibly could be depleted by 2100.
The hard truth is that the United States, Japan, and the
affluent industrial nations of Western Europe import much of their key minerals
from other countries-primarily the less developed nations. (Exceptions are the
U.S.S.R. and mainland China.) The dependence of the United States on other
countries for necessary minerals will increase dramatically: within the next
decade we will have to import more than half of the 12 key industrial minerals.
As we learned earlier, in natural ecosystems the Game of the
Environment continues because all materials needed for production are cycled
within the system. But we have not yet learned this important lesson.
We play the Game as if our mineral resources were infinite. Only a small
fraction of the material we use is recycled. Earthmanship - playing
the Game well-must be improved so that all necessary materials are
recycled as fully as possible.
But even if recycling were perfect, it still would not
provide the answer to our growing needs. Some mining would be required
simply to replace materials lost to rust, corrosion, or wear. Along with a
total commitment to conservation and recycling, we must give priority to
improving mining technology, locating new reserves, and developing substitute
materials.
But the problems encountered in playing the Game will be far
from solved even if all of these activities are successful. While
considerably less energy is required in recycling materials than in wresting
them from virgin sources, the increasing rate of consumption means that
ever increasing amounts of energy must be used. And energy cannot
be recycled - it is a noncyclic resource.
We do not have enough energy to extract resources, convert
them to products, and then recycle them at increasing rates.
Energy reserves
Is there really an energy crisis? Are fossil fuel reserves being depleted?
How soon will we run out?
Although-coal has been burned as fuel for more than 800
years, it is only since the early 1800's that sizable amounts have been
consumed. Fantastic increases in coal consumption accompanied the
Industrial Revolution (250 million tons in 1870 to 2.8 billion tons in 1970);
this dramatic increase occurred at the same time that the importance of coal was
declining sharply in favor of oil and natural gas.
Page 119
Indeed, oil production has doubled every 10
years since about the turn of the century (fig. 48). In the United States,
oil production peaked around 1970, and we have been increasingly dependent on
imports since. It is projected that world consumption of oil in this
decade (1970 to 1980) will equal that used during the previous 100 years!
Since we know that fossil fuel supplies are finite, the question of how long
they will last is critically important.
The most optimistic estimates are that reserves of natural
gas and "cheap" oil will be depleted (80 percent of total reserve used) by 2000
in the United States and about 30 years later in the rest of the world.
Figure 48. -
Massive oil fields on land and offshore, refineries, tankers, and tank farms point to our great dependency on oil and natural gas as major energy sources. It has been estimated that for all practical purposes, our reserves of natural gas and oil will be gone by the year 2000.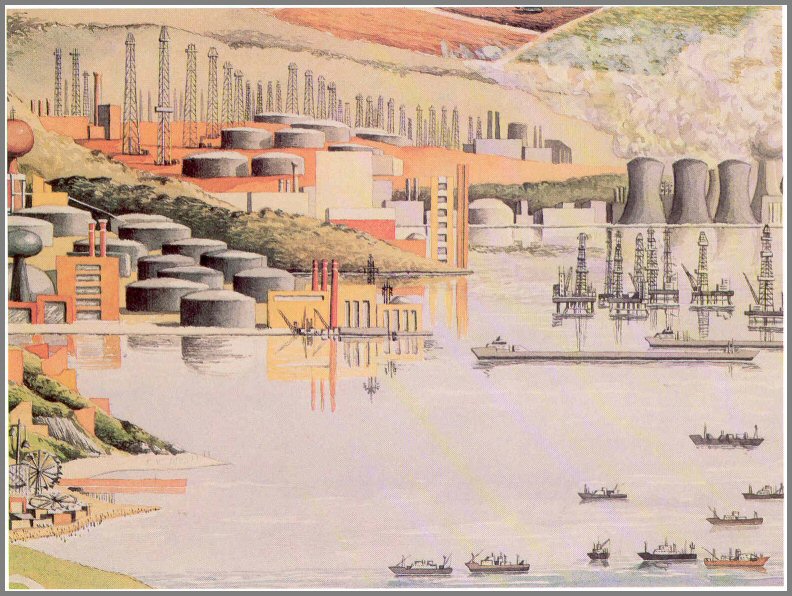
Page 120
The great hope for future sources of fuel seems to lie in our
coal reserves (perhaps important for 200 to 300 years, but only 75 years if coal
is the sole source of energy), and possibly in our vast deposits of western
oil-bearing shales. But "switching back to coal"
signals a potential increase in air and water pollution. Land reclamation,
air pollution controls, safety measures, and shipping will generate additional
costs.
And unlike its cost in the past, coal will be expensive.
Gassification- producing synthetic natural gas (SNG) from coal-has been proposed
as the answer to the high cost of shipping great quantities of coal from the
West to the East. But SNG is much less efficient than coal or natural gas,
and the necessary increasing in mining will deplete coal reserves sooner.
Similar disadvantages are associated with the exploitation of
shale oil. The product that is sought, kerogen, is present in such
low concentrations that huge quantities of rock must be ground and heated.
The great amount of energy required for extraction and shipping will result in a
low net amount of energy. And the impact on the environment is potentially
very great. Great tracts of land will be disrupted and vegetation and
wildlife destroyed. The quality of air and water will drop, as will water
tables; and because the crushed spoils will occupy more than 10 percent more
space than the solid rock, the waste disposal problem will be staggering.
These imminent shortages and escalating costs of fossil fuels
have led us to focus on nuclear fuel for generating power. But the process
currently used- fission of a relatively rare resource, uranium-235 - is very
wasteful. Uranium- 235 makes up less than 1 percent of the uranium in
natural ore. In fact, proponents of nuclear power fear that medium-priced
supplies of uranium-235 may be exhausted before breeder reactors are developed
to "breed" or make fission- able plutonium-239 and uranium-233. Either of
these isotopes can be used as a catalyst to burn uranium-238 or thorium-232,
which together represent an energy source millions of times larger than all
known reserves of fossil fuel.
It would seem, then, that nuclear breeder reactors, and
perhaps fusion reactors, offer the greatest hope for satisfying our insatiable
appetite for energy. Unfortunately, as with fossil fuel sources, there are
potentially serious consequences associated with nuclear energy-most of which
relate to environmental pollution.
The possibility that human or technological error will result
in a serious malfunction will increase as the number of reactors increases.
This is perhaps sufficient reason to engage now in an integrated national energy
program to develop technologies for alternative energy sources, especially
solar, wind, tidal, and geothermal power. While nuclear energy probably
will be a major, if ;not the primary, source of power in the decades to come, it
would be techno- logical insanity to put all our energy "eggs" in one
basket-especially in the one that poses unparalleled potential for biological
hazard.
Page 121

Page 122-123
Pollution
Pollutants are materials injected into the biosphere in
sufficient quantities to change the Game and to adversely affect the living
Players, especially people (see picture on p.122). For convenience,
pollutants are often classified as soil, air, or water pollutants, as
biodegradable or nonbiodegradable, or as threshold (damaging at some level) or
nonthreshold (damaging at any concentration). But pollution should be
looked at as a whole, because what begins as an air pollutant often ends up in
soil or water; and concentrations of substances damaging to one life stage of a
Player organism may not be harmful to other stages or kinds of organisms.
Nature can be a polluter! Volcanoes, earthquakes, dust
storms, and salt spray from ocean storms are major sources of natural pollution.
Even wildfire and floods, whose effects may be beneficial, can contribute to air
pollution or to undesirable siltation. But the intermittent and dispersed
nature of these natural Fouls lessens their impact on the environment.
Figure 49. - Too much phosphorus can trigger algal blooms in fresh water ecosystems, which, when extreme, can result in fish kills.

Page 124
People are the primary cause of pollution. Our actions as powerful biogeochemical agents-gathering, extracting, moving, concentrating, and dumping-have repeatedly swamped natural systems with too much material. We also have introduced many compounds that are totally alien to natural systems. Many of these substances are not biodegradable, and they accumulate or magnify in food chains until they become toxic. The chlorinated hydrocarbons (DDT, PCB) and radionucleids (strontium-90, cesium-130) are examples of introduced synthetic materials that now are distributed in significant quantities throughout the atmosphere. Thus, people can cause material cycles to "run amuck" by injecting into them excessive quantities of both natural and synthetic substances.
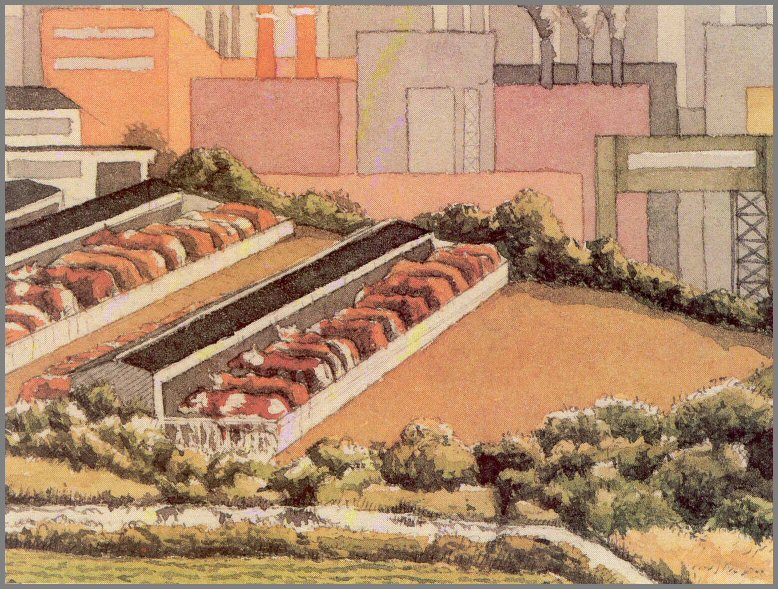
Figure 50. - Runoff from animal feedlots carries excessive amounts of phosphates and nitrates into waterways.
Page 125
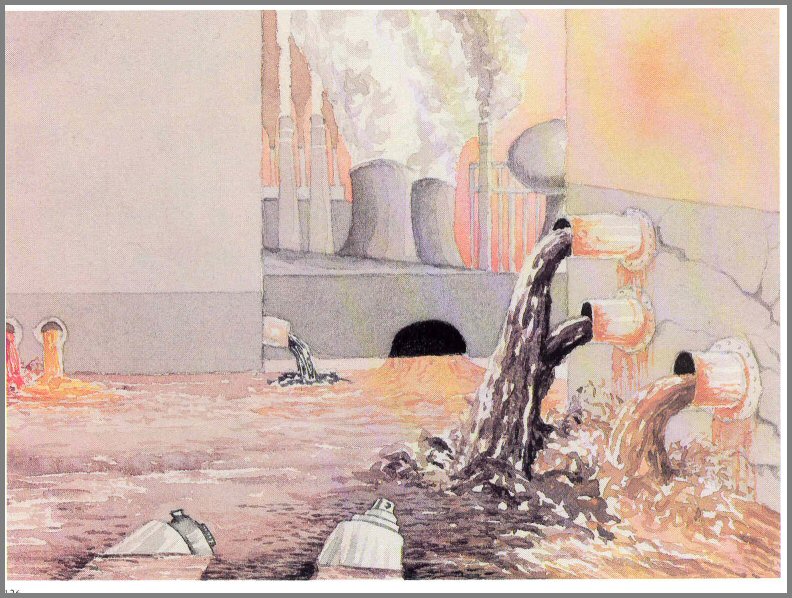
Figure 51. - Sewage rich in detergents is a primary source of phosphate pollution.
Page 126
Some cycles run amuck
Phosphorus - Phosphorus is
usually the element in short supply - the limiting factor-for growth of
algae in fresh water. The rich green blooms of algae common in many of our
rivers and lakes usually are signals that substantial amounts of phosphorus have
been added to the system (fig. 49). Most of this excess phosphorus enters
the cycle in treated or untreated sewage rich in detergents, and in runoff from
animal feedlots (figs. 50 and 51).
The growth of algae Producers often proceeds unchecked until
the excess phosphorus is used up and once again becomes limiting; then, a dieoff
occurs. Because the decomposition of large amounts of dead algae requires
large quantities of dissolved oxygen, fish may be killed.
Once phosphates reach estuarine or coastal Arenas, they no
longer contribute to algae blooms; in these systems, nitrogen is the limiting
factor. The suggestion that phosphate detergents be replaced with
nitrogenous ones must be evaluated carefully because this substitution might
seriously compound problems associated with explosions of phytoplankton and
zooplankton in ocean ecosystems where nitrogen is the limiting factor.
Page 127
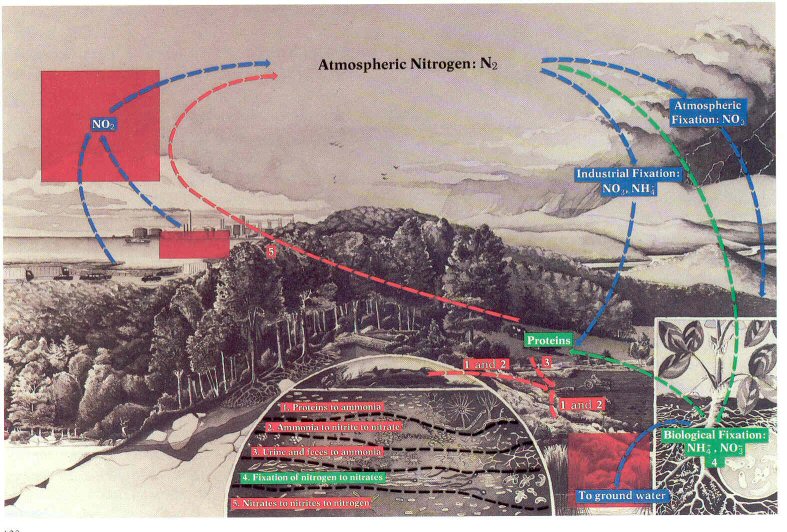
Figure 52.- As a result of human activities, the nitrogen cycle runs amuck {three boxes}: In the atmosphere when nitrogen oxides help to form ozone and smog; and in soil and water when nitrate-rich effluents speed eutrophication.
Page 128
Nitrogen. -Nitrogen monoxide (NO)
and nitrogen dioxide (NO2) are the
two of the eight nitrogen oxides that contribute to air pollution. These
gases are formed by the combustion of fossil fuels in automobiles and power
plants (fig. 52). Combustion at high temperatures and pressure converts
gaseous nitrogen to nitrogen monoxide and this, in turn, is oxidized rapidly by
ozone (O3) or slowly by oxygen to
nitrogen dioxide. Nitrogen dioxide is reduced by ultraviolet light to
nitrogen monoxide and atomic oxygen (O). The atomic oxygen can react with
oxygen to form ozone or with unburned hydrocarbon emissions to form
photochemical smog.
Nitrogen dioxide, ozone, and smog are harmful to plants and
animals; they cause irritation of the eye, nose, throat, and respiratory tract,
and they damage food crops and forests. The interaction of these
pollutants with others, especially with carbon monoxide and sulfur dioxide, can
cause great damage at relatively low concentrations.
As is shown in figure 52, the nitrogen cycle is carried out
not only in the atmosphere but also in soil and water. As with phosphorus,
excessive amounts of nitrogen in aquatic systems can result in overproduction-in
cultural eutrophication. And the polluting of aquatic systems with
overloads of nitrogen is increasing. Heavy losses of nitrate fertilizers
from agricultural systems often result from runoff, especially in humid
climates. Animal manure is potentially a primary source of nitrate
pollution, especially near feedlots (fig. 50); fortunately, most feedlots are
located in regions with low rainfall. One of the greatest sources of
nitrate pollution is human effluent discharged directly from waste treatment
facilities to waterways.
Page 129
Figure 53. - We disrupt the sulfur cycle by injecting great quantities of sulfur dioxide (SO
2) into the atmosphere (box). Sulfur dioxide combines with moisture in the atmosphere to form sulfuric acid-a compound that can be harmful to animals and plants.
Page 130
Sulfur dioxide. - We also are
affecting the sulfur cycle by introducing great quantities of sulfur dioxide (SO2)
into the atmosphere (fig. 53). Sulfur dioxide usually is only a transitory
step in the cycle, occurring in very low concentrations. Additions through
combustion and refining of sulfur-bearing fossil fuels and smelting account for
only about 20 percent of the total global amount (80 percent is from natural
sources); however, concentrations of SO2
in urban - areas are causing serious problems.
Once it is in the atmosphere, SO2
reacts with moisture to form sulfuric acid. When inhaled as a fine mist,
or when attached to small particles, sulfuric acid can injure sensitive lung
tissue; it is a major cause of bronchial asthma during air inversions. Low
concentrations of SO2 can injure and
even kill many important crop plants, especially when it occurs with low
concentrations of ozone.
Sulfur in the atmosphere produces acid precipitation
in many areas. Downwind from industrial centers, the acidity of rainfall
has increased up to 200 times in recent years. Sulfuric acid in the
atmosphere damages paint, stone buildings, sculpture, and ancient artifacts.
And the acidity of streams, sometimes great distances from the industrial
sources, has also increased, harming fish and other aquatic life. Although
the long-term effects of acid precipitation on terrestrial ecosystems are not
well understood, this phenomenon emphasizes strongly that our activities in one
location can adversely affect life processes many miles-even continents-away.
Heavy metals. - The cycles of many
elements that are not essential for growth also have been adversely affected by
people. The heavy metals -lead, mercury, and cadmium-that have always been
present in low levels have been injected into the biosphere in large quantities
by the burning of leaded gasoline, by smelting and other industrial processes,
and by the use of pesticides. These elements magnify in food chains and
accumulate in the blood and tissues of Consumers (including humans) at higher
trophic levels, where they can cause severe neurological symptoms, chromosome
breakage, and death.
Page 131
Hydrocarbons (chlorinated). -The
book Silent Spring by Rachel Carson alerted the public to the dangers of
chlorinated hydrocarbon pesticides. The global spread of DDT and its
unsuspected and dramatic impacts on nontarget organisms in distant areas point
out the dangers of injecting huge quantities of synthetic materials into the
biosphere (fig. 54).
The very characteristics of toxicity, persistence, and
stability that made DDT attractive as an insecticide account for its spread and
adverse effects. Insoluble in water but highly soluble in fats and oils,
DDT accumulates in fatty tissues of organisms. It does not break down
easily, and it continues to magnify in food chains. Indeed, a study along
the northeastern Atlantic coast revealed the level of DOT in gulls to be a
million times more concentrated than it was in the water!
Figure 54. -Synthetic compounds, like the chlorinated hydrocarbon pesticides, become incorporated into biological cycles. When they magnify in food chains, they can be lethal to nontarget organisms great distances away.

Page 132
Death or impaired reproduction can result when organisms
acquire high concentrations of DDT. Small fish can be killed from large
doses of DDT that are stored in their yolk sacs; DDT in birds alters their
calcium metabolism, resulting in death directly or indirectly when
calcium-deficient egg shells break during incubation.
The greatest danger from DDT and similar compounds may not be
from direct exposure but from subtle changes in the structure and function of
the Game of the Environment. When beneficial nontarget organisms are
killed by pesticides, food chains become shortened- simplified,- and the Game is
weakened. Materials that are substituted for chlorinated hydrocarbons are
usually shortlived-they do not magnify in food chains. But the extreme
toxicity of some of these substitutes poses real dangers through mishandling by
humans.
Other synthetic compounds have attracted attention in recent
years. Polychlorinated biphenyls (PCB's) used widely in industry are
perhaps more dangerous than DDT. PCB's-also persist and magnify in food
chains and kill many predaceous organisms.
Page 133
Figure 55. - Oil spills are dramatic examples of pollution, but most oil pollution is not nearly as visible.

Page 134
Hydrocarbons (oil). -Oil pollution
can be a dramatic event (fig. 55). Headlines tell of jumbo tankers
breaking up, of offshore well blowouts, and of the potential failure of newly
constructed pipelines. Such events reflect our increasing appetite for
oil. As dwindling supplies necessitate exploiting reserves in increasingly
inhospitable Arenas, the likelihood of serious accidents increases. But
most oil pollution (80 to 90 percent) occurs during everyday shipping, refining,
processing, and burning of hydrocarbons. The most important form of oil
pollution is fallout of airborne hydrocarbons.
When hydrocarbons reach the oceans, they are diluted and
dispersed. Eventually, they disappear through microbial degradation,
evaporation, oxidation, and deposition. Along the way, however, great
damage may occur to ocean- life. Sometimes this damage is obvious,
sometimes it is subtle and indirect. Thus, hydrocarbons can destroy vital
parts of some food chains directly (for example, aquatic insects), can
accumulate and magnify in food chains (for example, in large fish and birds near
tops of food chains), and can interfere with the communication systems of
organisms (for example, disruption of chemical "messages" from rivers to fish
returning to spawn).
Page 135
Figure 56. - Today, the greatest threats of radiation pollution stem not from nuclear fallout, but from radioactive wastes created in atomic power plants.

Page 136
Radioactive materials.
-Atomic bombs and the testing of nuclear weapons ushered forth
the atomic age and the threat of radiation pollution (fig. 56). This
threat is very great. The terrible radioactive fallout from massive atomic
explosions is well known. But the dangers from radioisotopes that may
enter biological cycles are also potentially great.
Radiostrontium, the product of uranium fission, is a good
example of a material that can cause a cycle to subtly run amuck.
Strontium "mimics" calcium and it can eventually become incorporated with
calcium in human bones. Here, it is in close contact with blood-forming
marrow-tissue that is especially sensitive to radiation damage.
Genetic damage to reproductive cells, with the threat of
passing on mutations to unborn generations, can also occur from radiation.
"Safe" levels are difficult to establish; the harmful effects of chronic
exposure to low levels of radiation are still being studied.
There is little doubt that the greatest threat of pollution
originates in transporting, storing, and reprocessing spent radioactive wastes.
Large quantities of these wastes are now being stockpiled for reprocessing.
They are a major liability; this material must be maintained-with no
mistakes-for thousands of years. Wastes of other kinds, although posing
less imminent threats, nevertheless affect our well-being as they interfere with
biogeochemical cycling.
Page 137
Figure 57. - Disposing of billions of tons of solid waste each year is costly in terms of land, money, and mineral resources.

Page 138
Solid Waste. -Solid waste is
increasing, especially in urban areas (fig. 57). Billions of tons are
produced each year, and billions of dollars are spent in collecting and
disposing of it. And disposing of solid waste is becoming more and more
difficult. Poorly designed landfills can pollute land, water, and air, and
the incineration of this material can contribute significantly to air pollution.
Space is often unavailable for landfills near urban areas where huge quantities
of trash must be disposed of each day. But the most serious and important
aspects of disposal by burning or burial are the drain on our mineral resources
and the loss of "waste" land such as wetlands and marshes.
Sewage. - The treatment of wastes
by most of our sewage treatment plants involves only the primary or secondary
phases or both. In primary systems, solids are screened and sedimented
from waste water and then burned or buried. Secondary treatment consists
of biological degradation of organic matter. The cheapest secondary system
is a shallow oxidation pond where algae provide the aeration; mechanical
aeration can speed up this process.
However, secondary treatment does not remove nutrients from
sewage. When nutrients, such as phosphorus and nitrogen, discussed
previously under "Some cycles run amuck," are transported back to natural
Arenas, there must be sufficient space and food chains to handle them or
pollution will result.
The expensive tertiary or advanced treatment includes
the removal of phosphates, nitrates, organics, and other substances. More
and more interest is being expressed in using terrestrial ecosystems as tertiary
treatment systems.
Page 139
Figure 58. - When too much heat, liberated into air or water, causes adverse effects, it is a pollutant.

Page 140
Messing up the flow of energy
Heat.
-In figure 58, the cooling towers of an atomic power plant dominate the scene. This is appropriate because one of the greatest pollutants created by human activities is heat.Page 141
Too much heat in aquatic Arenas can be harmful to the living Players. They can be:
Killed directly by a sudden change in
temperature.
Rendered susceptible to parasites and
diseases.
Starved for oxygen.
Starved for food because lower levels of
their food chains were destroyed.
Disrupted in their patterns of migration.
Overall, aquatic ecosystems can be degraded as eutrophication is speeded up, and as species composition changes to fewer and less desirable species.
The picture on pages 144 and 145 shows an ideal system
where we, as the central Players of the Game of the Environment, have attuned
ourselves and our actions to accommodate the Rules. Only a few of the
myriad ways to improve the Game are shown -"but all of these methods are
available now or are easily within technological reach, and many of them
are economically feasible (perhaps even profitable).
This illustration provides a sharp contrast to those that
precede it. It presents the view that cities will remain the focal
points of human culture, and that we will continue to dominate and
exploit our environment. But it also points out the hope that this
exploitation will take place within the Rules that govern our
environment.
Page 142
---- ---- ---- ---- ----
IN YOUR FUTURE ENVIRONMENTAL ARENA
Compare the illustration on page 144 with illustrations on pages 106,114,
and 122; list the similarities and differences between this illustration and
each of the others.
Page 143

Page 144-145