Water and Trees
By Dr. Alex L. Shigo
Water and energy are twins. In many parts of the world, supplies
are decreasing as demands are increasing. Wise management -starting with
education -is the answer to this potential problem.
 Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
Water, water, everywhere, but only 0.05 percent to drink! Oceans
cover 71 percent of the earth's surface, but ocean water is too salty for
people and trees. Many plants and a few species of trees do live in salty
water. The salty water makes up 97 percent of the earth's water. Of the
remaining fresh 3 percent, 75 percent is in ice at the poles. The rest
can be used for drinking. However, most of it is inaccessible ground- water.
We are back to 0.05 percent available to us from lakes and streams. We
not only drink it; we wash in it, flush it, and use it for irrigation of
grass, crops and trees as if it will never run out. In many places in the
world, it has run out. As trees were cut the land heated. No clouds formed.
No rain fell.
 Water is
held in clay soils. This can be very beneficial when in moderate
amounts, but when too much water saturates the clay, problems start - usually
root rots.
Water is
held in clay soils. This can be very beneficial when in moderate
amounts, but when too much water saturates the clay, problems start - usually
root rots.
Water, trees and life
Arborists know about water best by its amounts in extremes: too much,
too little. Too much brings floods, or when frozen, breakage. Too little
brings droughts. Amounts of precipitation are out of human control. Humans
do bring on tree problems when they water too much, or forget to water.
Stress is a condition where a system begins to operate near the
limits for the way it is designed. Water is an essential for all life systems
to survive. When too much or too little water is present, the tree system
begins to operate near its limit for survival. Stress. Water - caused stress
is a major predisposing factor for a long list of tree problems that could
end in death. Root problems are at the top of the list. Insects and fungi
are easy to see and they will always be there. Fighting secondary agents
of tree problems has become the primary role of many people. Water as a
liquid dissolves many substances essential for the life of trees. Water
transports the substances throughout the tree. Water is essential for photosynthesis
and its end product, glucose. As bound water, it acts as a storage product.
The way water changes from free to bound, and back again, is one the wondrous
processes of nature.

A
Plea for Modern Arboriculture
Years ago I predicted that in the 21st century, arboriculture would
begin to split as more arborists moved from old arboriculture toward modern
arboriculture. Old arboriculture will not go away. It is, and will be for
many years, the dominant force for tree care.
New people are coming on the scenes, and the scenes, or demands of
the marketplace, are changing rapidly. Survival of any individual or system
depends directly on their ability to adjust to changes. The rate of adjustment
defines the winners. Some arborists believe that chemistry is not arboriculture,
and that it has no place in arboriculture. A few teachers have told me
they do not use my book, Modem Arboriculture, because it contains some
very simple chemistry, which is not arboriculture. Many teachers do understand
chemistry but their schedules do not allow time to teach it. But, what
about the arborists who are sick and tired of the same old stuff?
They want something new and better. It will take time to bring modem arboriculture
into full bloom. A better understanding of tree biology and chemistry is
the basis for modem arboriculture. Sad, but biology and chemistry still
frighten many people. Here I give a brief glimpse of water, one of the
most essential substances for trees and for all living things. To be an
arborist and not have some understanding about water is unthinkable for
me. I'm sure some arborists will not read this article. I'm also sure that
others will not only read it, but chew it and study it. If you want more
of this stuff, I should be pleased to give it. If not, so be it. I respect
trees and arborists. I believe they deserve and need something new and
better, not the same old stuff.
What is water?
Water is a substance in which two hydrogen atoms bond in a unique way
to one oxygen atom, hence, H2O. The unique bonding is so spectacular that
water takes on fascinating characteristics. It is the only substance on
earth that occurs naturally as a liquid, gas or solid. All water on earth
originally came from rocks. As the extremely hot, young earth began to
cool, gases such as oxygen and hydrogen escaped from rocks. They collected
above the earth, and as some oxygen and hydrogen bonded, the rains came.
Your basic atom
Atom was the name given to the smallest bit of matter. The word means
uncuttable. Of course we know now that atoms can be reduced or cut further.
There are 92 naturally occurring kinds of atoms. In elaborate laboratories,
scientists have increased that number to 110, as of this writing.
An atom contains at least one central, positively charged body and
one circling, negatively charged body. Every atom is unique in that the
number of positive charges normally equals the number of negative charges.
The positive bodies are protons, and the negative bodies are electrons.
The circling nature of the electrons is often referred to as a negative
cloud. All atoms except hydrogen have at least one neutron in their nucleus.
The neutron has mass, but no charge. The hydrogen atom has one proton and
one electron, but no neutron.
If the nucleus of an atom could be enlarged to about the size of
a dime, the circling cloud of the electron or electrons would be nearly
the size of a football field. Think about it. A half -inch cube of nuclear
material would weigh about 10 million tons. The figures lose their meaning
because it is difficult for our minds to grasp these facts. In the end,
we must remember the energy and matter are concepts, and that they are
interchangeable.
More about hydrogen
Hydrogen is the most abundant atom in the universe. Because of its abundance
on the sun, there is life on Earth. On the sun, the heat and pressures
are so great that hydrogen atoms are fused to form helium atoms. In this
fusion process, some matter is converted to enormous amounts of energy.
The energy radiates from the sun as light. Chlorophyll in trees and other
green plants traps some of the light energy that is ultimately used to
form glucose. Carbon dioxide and water are key players in this process.
This may be why water is often called the substance of life.
Hydrogen starts the many events that lead to water, energy and life.
Hydrogen is a unique atom because it normally does not contain a neutron.
To understand the ways of hydrogen' s single proton and electron is to
understand much about chemistry , life and, here, trees.
The single electron rotates about the proton in a cloud that is
commonly called a ring. The single ring of hydrogen could accommodate two
electrons. But, if it did, this would unbalance the charges, and this won't
happen unless something forces it to. Normally the number of protons equals
the numbers of electrons.
Models have been developed for atoms so that discussions about them
could be easier. In the models, the first ring could have two electrons,
and the second ring eight electrons. Of course the "real" nature of the
atoms are replete with exceptions and strange characteristics. However,
with water and hydrogen and oxygen, most of the model terms are applicable.
More about oxygen
Oxygen has eight protons and eight neutrons in its nucleus, and
eight electrons in two rings. The first ring is saturated with two electrons
and the second ring has six. It can hold eight electrons.
We breathe oxygen so it can combine with hydrogen "left over" from
our energy- yielding processes. When it does connect or bond with hydrogen,
we breathe it out as water vapor. It seems that we just cannot get away
from water and life, and in this case, our own life.
Water is also essential for the life of trees, and trees provide
arborists with the means of their life and business.
Oxygen is a product of photosynthesis. We say oxygen is given off
to the air. In the process of photosynthesis - where carbon dioxide and
water are the ingredients - the power for the process comes from the hydrogen
in the water. In a sense, water is split, or to be even more precise, the
protons and electrons of the hydrogen atoms are separated. After many chemical
processes, oxygen is released.
Oxygen becomes very essential in respiration. In this process,
the energy stored in glucose is released to do the work of life. The products
of respiration are carbon dioxide and water. Back to water again.
The processes of photosynthesis start with carbon dioxide and water,
and, in the end, the processes of respiration end with the release of carbon
dioxide and water. In all of this, the power of the sun is used to make
life on Earth possible. Oxygen, carbon dioxide and water are
the actors.
They start and they finish still being the same actors ready to act again
and again for continued new life.
Now if all of this does not "grab you" then there is no hope !
Bonding patterns
Atoms bond with other atoms to form dogs, cats, humans and trees. All
life forms are made up of atoms bonded in unique ways, often in the form
of electrically neutral molecules.
The strongest bonds are called covalent. With these bonds, two or
more atoms share electron fields by actually penetrating one another's
fields. The next level of bonding is called ionic. Each atom or group of
atoms here has a positive or negative charge. Such atoms or groups are
called ions. Because unlike charges attract, ions of unlike charges bond,
but do not penetrate each other's electron field. We commonly call many
of these ion combinations "salts." Common table salt is really a crystal
made up of sodium ions bonded to chloride ions. Table salt is not a molecule.
When the crystals are poured into water , the ionic bonds separate. The
same processes operate for commonly used fertilizers. They are salts. In
water, their bonds are released.
In the third type of bonding, the atoms or groups come fairly close
together, but do not touch. This bonding pattern is the weakest, yet this
pattern is the major one that holds you and trees together. On a relative
numerical basis, consider the holding power of these bonds to be about
two or three; on the same scale, the covalent holding power between two
nitrogen atoms in the air is about 190.
Yes, life forms are held together by these relatively weak bonding
forces. If this were not so, processes of breakdown and buildup would not
work. No recycling. No new life.
This third type of bonding brings us back to water, and its ingredients
- oxygen and hydrogen. The third type of weak bonding is called hydrogen
bonding. Because it is so important, some additional details should be
given.
Hydrogen bonds
Hydrogen bonds are the unique features of water. In summary, oxygen
has two positions for additional electrons in its second ring. Hydrogen
has one electron in its single ring, but the ring can accommodate two electrons.
Two hydrogen atoms bond with a single oxygen atom to form a molecule
called water. Each hydrogen atom bonds on the second ring of the
oxygen atom where there is a place for them. When the hydrogen atoms bond
with the oxygen atom, a strange partnership takes place. Each hydrogen
atom now has two electrons in its ring and the oxygen atom has electrons
filling the two available positions on its second ring. Add to this the
fact that the hydrogen atoms and the oxygen atom now have their rings saturated,
yet the positive and negative charges of the molecule are balanced! What
a process!
There is much more to this story of water. Oxygen "accepts" the
electrons of the hydrogen atoms, but it pulls most of their electron clouds
deep into its atom. Another way to say this is that the electrons of the
hydrogen atoms spend much more time deep inside the oxygen atom's ring
than they do rotating about the protons in the hydrogen atoms.
The hydrogen protons as a result are near the outer edge of their
ring, with very little electron negative charge about them. The protons,
being positive, exert their charges out from their position on their rings.
And, because the oxygen has absorbed most of the negative charges
of the electrons of the hydrogen atoms, the side opposite the hydrogen
atoms becomes weakly negative. So now one part of the water molecule has
two weak positive points and the opposite side two weak negative points.
Such a molecule is called a dipole. Water is a dipole.
Here is another way to view the water molecule. Imagine oxygen as
a large clear ball. Now, mark four points on the ball all equidistant from
each other. Make two points red and two green. Next, move the green points
slightly away from each other, and move the red points slightly toward
each other the same distance that you moved the green points. The two green
points have weak negative charges, and the two red points have weak positive
charges. The red points are positions where the hydrogen atoms are bonded
to the oxygen. The exact points of red are the positions where the protons
reside and are producing the weak positive charges. If you can imagine
this three - dimensional model of water in your mind, many fascinating
characteristics of water become easy to explain and understand.
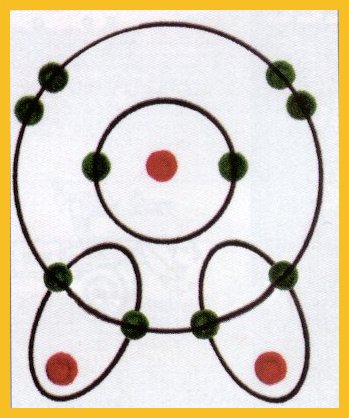
 Figure
1 : Oxygen, above, has eight electrons in two rings, and eight protons
and eight neutrons in its nucleus. Shown here are two - dimensional diagrams
for three - dimensional atoms and molecules. All diagrams are from models
and the nucleus and electrons are greatly enlarged. (Red = Positive; Green
= Negative) The hydrogen atoms, below, each has a single proton and single
electron in one ring.
Figure
1 : Oxygen, above, has eight electrons in two rings, and eight protons
and eight neutrons in its nucleus. Shown here are two - dimensional diagrams
for three - dimensional atoms and molecules. All diagrams are from models
and the nucleus and electrons are greatly enlarged. (Red = Positive; Green
= Negative) The hydrogen atoms, below, each has a single proton and single
electron in one ring.
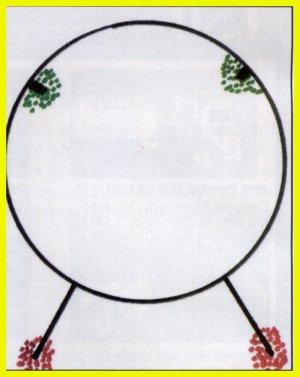
 Figure
2: Water forms when two hydrogen atoms bond with an oxygen atom.
Weak positive charges extend from the protons in each hydrogen atom, and
two weak negative charges extend from the opposite side of the molecules.
Figure
2: Water forms when two hydrogen atoms bond with an oxygen atom.
Weak positive charges extend from the protons in each hydrogen atom, and
two weak negative charges extend from the opposite side of the molecules.
 Water is
used in abundance to maintain lawns, garden and trees in some of the driest
parts of the world.
Water is
used in abundance to maintain lawns, garden and trees in some of the driest
parts of the world.
 Water and
trees were both at the Khyber Pass many years ago, I have been told.
Now, neither are present. The question is, what part did the removal
of the trees play in the problem?
Water and
trees were both at the Khyber Pass many years ago, I have been told.
Now, neither are present. The question is, what part did the removal
of the trees play in the problem?
Cohesive water
Water forms drops as it rains and falls on leaves and needles. If water
is poured on a smooth glass surface, mounds will form. If you pour alcohol
on the same surface, no mounds will form. Why? The answer: water has an
abundance of hydrogen bonds; alcohol does not. Back to our ball model.
You can bond one water molecule with another molecule, or even bond four
molecules with one molecule. However, you cannot have one water molecule
bond its two positive sites with the two negative sites on another water
molecule. Remember, the red dots are closer together than the green dots.
You cannot fit two red dots over two green dots.
Back again to four on one. It is possible for four molecules of
water to align themselves in such a way that they bond one of their dots
with a dot of a different color on the ball. As each molecule moves into
position where its positive site bonds with a negative site of another
molecule, an active dance goes on. If you can imagine it, every water molecule
is "trying" to bond with another. The problem starts for the molecules
when bonding partners position their other sites too close to similarly
charged sites on the molecules. Remember, unlike charges do attract, but
like charges repel. And, because the hydrogen bonds are such weak bonds,
it does not take much to knock them apart. So, the wild dance goes on as
molecules vie for positions only to be knocked out of place again and again.
The significance of this process for life and for trees specifically cannot
be overrated. Cohesion makes it possible for water to cling within vessels
and tracheids. The cohesive feature gives us raindrops and water as a liquid
at temperatures below 100 degrees Celsius. Many liquids, alcohol included,
form few hydrogen bonds. Ammonia, which weighs the same as water on a chemical
scale, is a gas at normal temperatures - again because its molecules do
not bond together as water molecules do.
Water from liquid to ice
As long as the dance goes on, liquid water exists. As temperatures begin
to decrease, the pace of the dance decreases until, at 4 degrees Celsius,
everyone gets a last chance to pick a bonding site. Because many of the
molecules that would normally be in the middle of the group now move to
outer positions to find a bonding partner, the volume or space occupied
by the dancers increases. We say that as water's temperature drops near
4 degrees Celsius, expansion takes place. As water expands, bottles or
even large rocks can be broken. The power of expanding water has been used
by humans down through history. As a result of further cooling, the
dance stops, as every molecule has a position. We call this state ice.
Because ice is less dense than an equal volume of water, it floats - all
because of hydrogen bonds. Some people have said that hydrogen bonds (icebergs)
caused the sinking of the Titanic. Yes, water can be good, and it can be
bad!
 Water in
its solid form (ice) is a major cause of tree fractures.
Water in
its solid form (ice) is a major cause of tree fractures.
Bound Water
How do trees stay alive in areas of the world where winter temperatures
are far below freezing? How do trees store water?
Every arborist needs to know something about those two questions,
mainly because many of the major cities of the world are in areas where
winters are cold. The simple answer again is hydrogen bonds. Let me explain.
Trees are made up mostly of cellulose. Cellulose is made up mostly
of glucose units bonded in ways that cause the units to twist as a rope
does. Water plays an important role here, but the details go far beyond
the scope of this article. Suffice it to say, the removal of a water molecule
between two glucose units results in the cellulose pattern. The twisting
takes place because the glucose units must be in a very precise position
to enable the water molecule's removal. My only point here is that water
does play a major role in the formation of cellulose. The free water becomes
available then to the tree.
Cellulose has many oxygen and hydrogen units as part of its makeup.
In a sense, the oxygen - hydrogen units "stick out" from the glucose -
now cellulose -molecule. Because each oxygen has a weak negative charge,
the site could be a potential bonding site for a positive charge from a
hydrogen atom that is part of water . The story continues with the same
theme. As liquid water comes in contact with cellulose, some of the positive
sites on the water molecule bond with the negative sites on the oxygen
atoms that are part of the cellulose. A hydrogen bond again. As more liquid
water comes into the same area, the water begins to bond with other water
molecules as it normally does. Remember: Cellulose - especially cellulose
in the middle layer of the second wall of fibers -is made up of many "ropes"
of cellulose with some spaces in between. The water molecules with their
hydrogen bonds soon start filling all the empty spaces. As the molecules
of water squeeze into every available space, spaces soon become saturated.
This point is called the fiber saturation point of wood, which is the point
where all available spaces are taken by water.
This is usually the normal healthy condition of trees. When this
condition exists, pathogens usually are not able to invade. So, water plays
a major role as a preventative against many pathogens.
When water is bonded to the cellulose, the water is called bound
water. Because it is bonded to the cellulose, it does not freeze as liquid
water does. Remember, the bonding power of the hydrogen bond is very weak.
It takes little to pull it apart. The bound water not only prevents freezing
and acts to prevent pathogens from invading; the bound water also is a
unique way for trees to store water .
 Figure
3: A two - dimensional diagrammatic view shows the negative (green) and
positive (red) charges on the water molecule. The negatively charged sites
are farther apart than the positively charged sites.
Figure
3: A two - dimensional diagrammatic view shows the negative (green) and
positive (red) charges on the water molecule. The negatively charged sites
are farther apart than the positively charged sites.
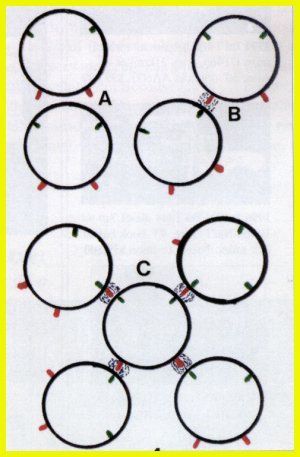 Figure
4:
Figure
4:
A: The diagrams of two water molecules show that the two negative
and the two positive sites do not align for bonding.
B: One water molecule can bond with another water molecule when
opposite charges are in direct alignment.
C: It is possible to have four water molecules bond with one
other water molecule when all oppositely charged sites are in direct alignment.
When such bonding arrangements bring like charges too close together, the
molecules move apart, but only to bond again at different sites. This repositioning
of molecules is responsible for water as a liquid.
From flush to free water
Trees store water as bound water and energy in starch and oils.
When the flush for new growth starts, some of the stored starch in living
parenchyma cells in wood and behind buds is converted back to glucose.
Water plays a role here also, because to go from insoluble starch to glucose,
a molecule of water must be chemically inserted back into each starch unit.
As this process goes on, the glucose dissolves back into the free water.
The glucose in the free water brings on a pull force that easily dislodges
more stored bound water. In fact, this process triggers the entire
process of liquid transport in trees. It starts the pumps. But, that's
another story about water .
“An author,
lecturer and consultant, Dr. Shigo started
Shigo and Trees, Associates
twenty
years ago after retirement from the U.S. Forest Service.”
Special thanks to Dr. Charles
Owens, professor of chemistry, for review of this paper and continuing
advice on chemistry.
Reproduced with permission of Tree Care Industry and Dr. Alex
L. Shigo.
The article was published in Volume XII, Number 2-February 2001
of TCI.
This site is dedicated to the remembrance of Robert Felix who
for many years worked very hard for the improvement of the tree care industry: 1934-1996.
Back to Articles.
Dictionary MAIN
PAGE
Text & Graphics Copyright © 2009
Keslick & Son Modern Arboriculture
Please report web site problems, comments and words of interest,
not found.
Contact
 Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
 Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
Water as
snow adds beauty to these beech leaves. As snow melts, the water seeps
slowly into the soil and run-off is minimized.
 Water is
held in clay soils. This can be very beneficial when in moderate
amounts, but when too much water saturates the clay, problems start - usually
root rots.
Water is
held in clay soils. This can be very beneficial when in moderate
amounts, but when too much water saturates the clay, problems start - usually
root rots.

 Figure
1 : Oxygen, above, has eight electrons in two rings, and eight protons
and eight neutrons in its nucleus. Shown here are two - dimensional diagrams
for three - dimensional atoms and molecules. All diagrams are from models
and the nucleus and electrons are greatly enlarged. (Red = Positive; Green
= Negative) The hydrogen atoms, below, each has a single proton and single
electron in one ring.
Figure
1 : Oxygen, above, has eight electrons in two rings, and eight protons
and eight neutrons in its nucleus. Shown here are two - dimensional diagrams
for three - dimensional atoms and molecules. All diagrams are from models
and the nucleus and electrons are greatly enlarged. (Red = Positive; Green
= Negative) The hydrogen atoms, below, each has a single proton and single
electron in one ring.
 Figure
2: Water forms when two hydrogen atoms bond with an oxygen atom.
Weak positive charges extend from the protons in each hydrogen atom, and
two weak negative charges extend from the opposite side of the molecules.
Figure
2: Water forms when two hydrogen atoms bond with an oxygen atom.
Weak positive charges extend from the protons in each hydrogen atom, and
two weak negative charges extend from the opposite side of the molecules.
 Water is
used in abundance to maintain lawns, garden and trees in some of the driest
parts of the world.
Water is
used in abundance to maintain lawns, garden and trees in some of the driest
parts of the world.
 Water and
trees were both at the Khyber Pass many years ago, I have been told.
Now, neither are present. The question is, what part did the removal
of the trees play in the problem?
Water and
trees were both at the Khyber Pass many years ago, I have been told.
Now, neither are present. The question is, what part did the removal
of the trees play in the problem?
 Water in
its solid form (ice) is a major cause of tree fractures.
Water in
its solid form (ice) is a major cause of tree fractures.
 Figure
3: A two - dimensional diagrammatic view shows the negative (green) and
positive (red) charges on the water molecule. The negatively charged sites
are farther apart than the positively charged sites.
Figure
3: A two - dimensional diagrammatic view shows the negative (green) and
positive (red) charges on the water molecule. The negatively charged sites
are farther apart than the positively charged sites.
 Figure
4:
Figure
4: